Click here to start your patients
on the path to just 2 DOSES A YEAR
- For US Healthcare Professionals Only

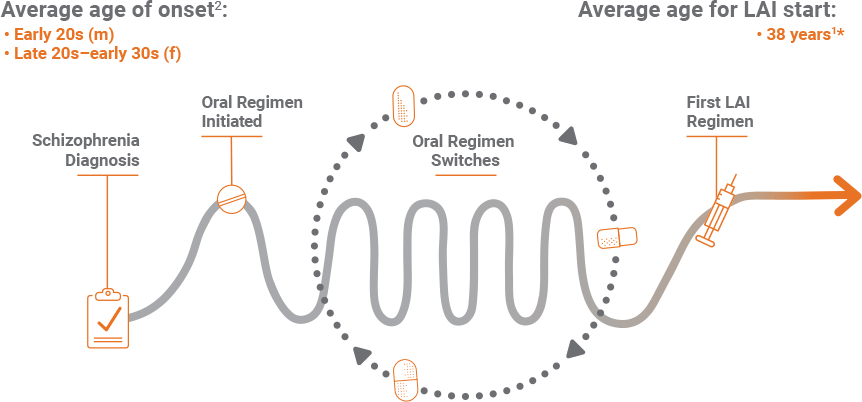
*From Janssen-sponsored market research, a total of 620 patient charts were collected from 161 healthcare providers (HCPs) from August 22 through September 12, 2016. Key screening criteria included: must have seen at least 30 individual patients with schizophrenia during the past 3 months, must have treated at least 1 patient with 1 of the 5 specified LAIs in the past 3 months, and should have been in practice for a minimum of 2 and a maximum of 35 years.
Clinically unstable or history of relapse

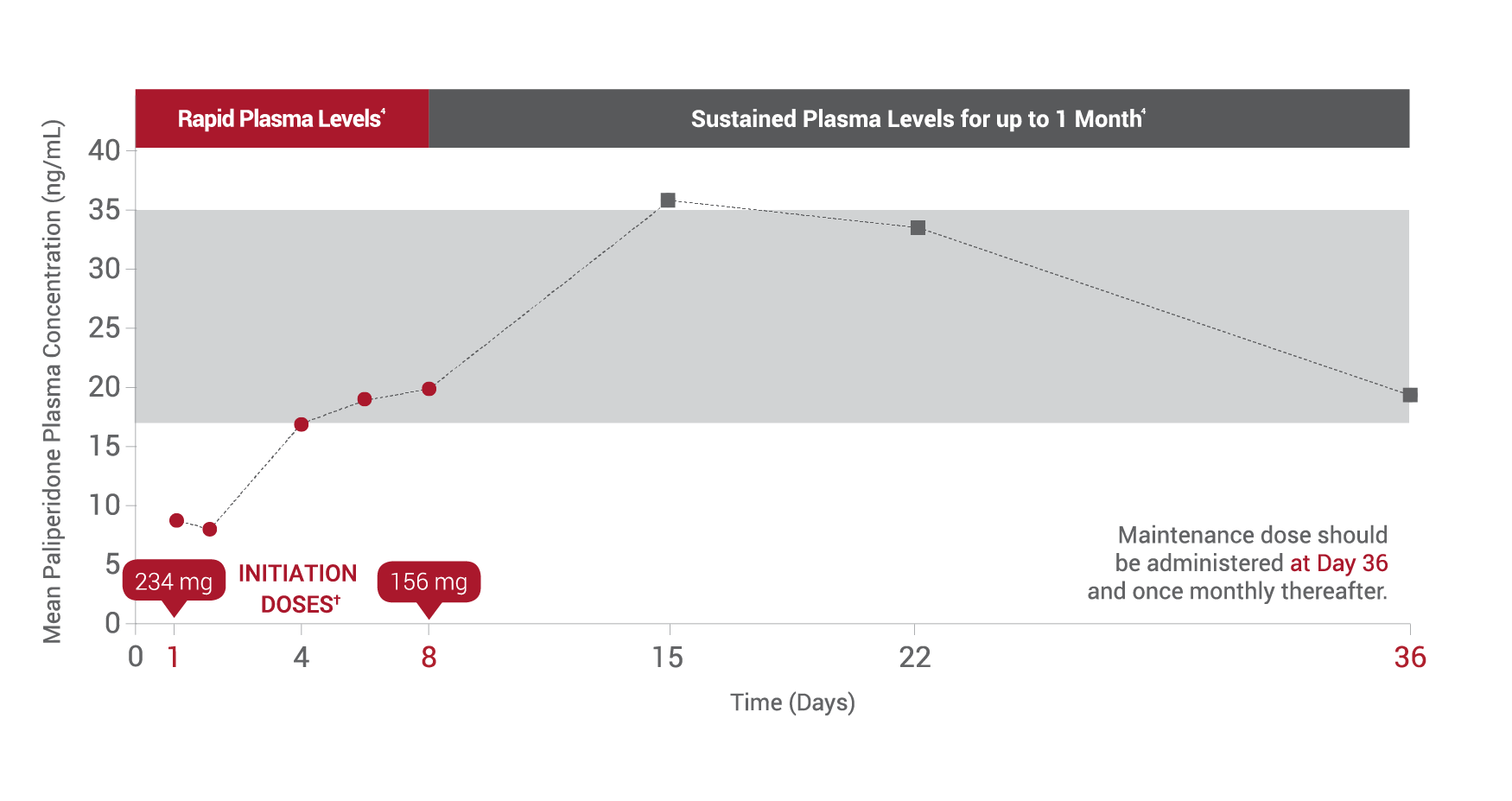
*After both initiation doses.
†Both initiation doses must be delivered in the deltoid muscle.
References: 1. Data on file. Chart Review. Janssen Pharmaceuticals, Inc., Titusville, NJ. 2016. 2. National Alliance on Mental Illness. What is Schizophrenia? Published November 15, 2013. Accessed September 14, 2022. https://www.nami.org/About-Mental-Illness/Mental-Health-Conditions/Schizophrenia
3. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; July 2022. 4. Samtani MN, et al. Dosing and switching strategies for paliperidone palmitate: based on population pharmacokinetic modelling and clinical trial data. CNS Drugs. 2011;25(10):829-845. doi:10.2165/11591690-000000000-00000