Cholesterol
From 4 placebo-controlled, 9- to 13-week, fixed-dose studies
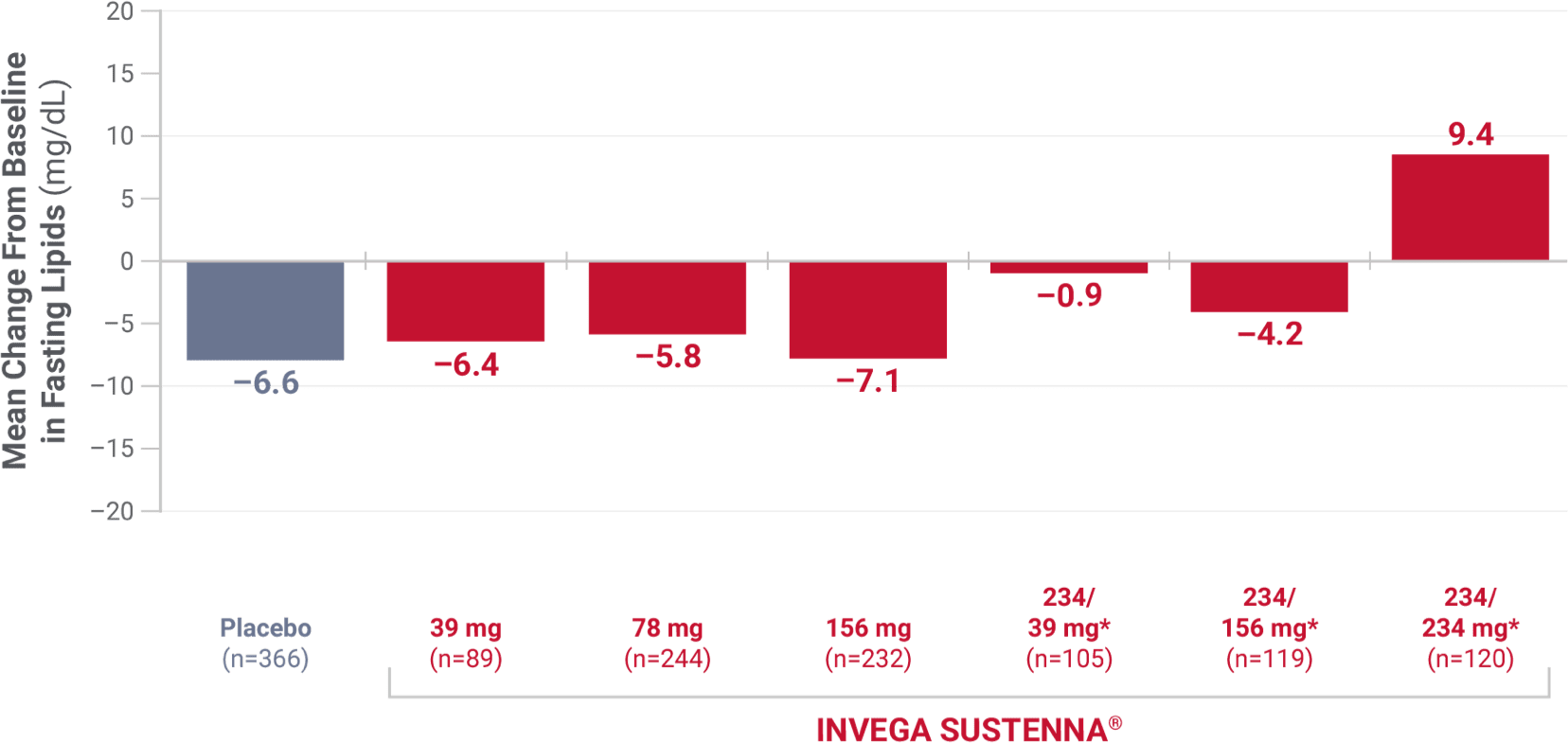
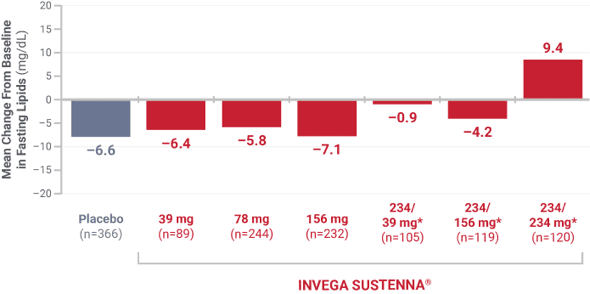
Low-density lipoprotein (LDL)
From 4 placebo-controlled, 9- to 13-week, fixed-dose studies
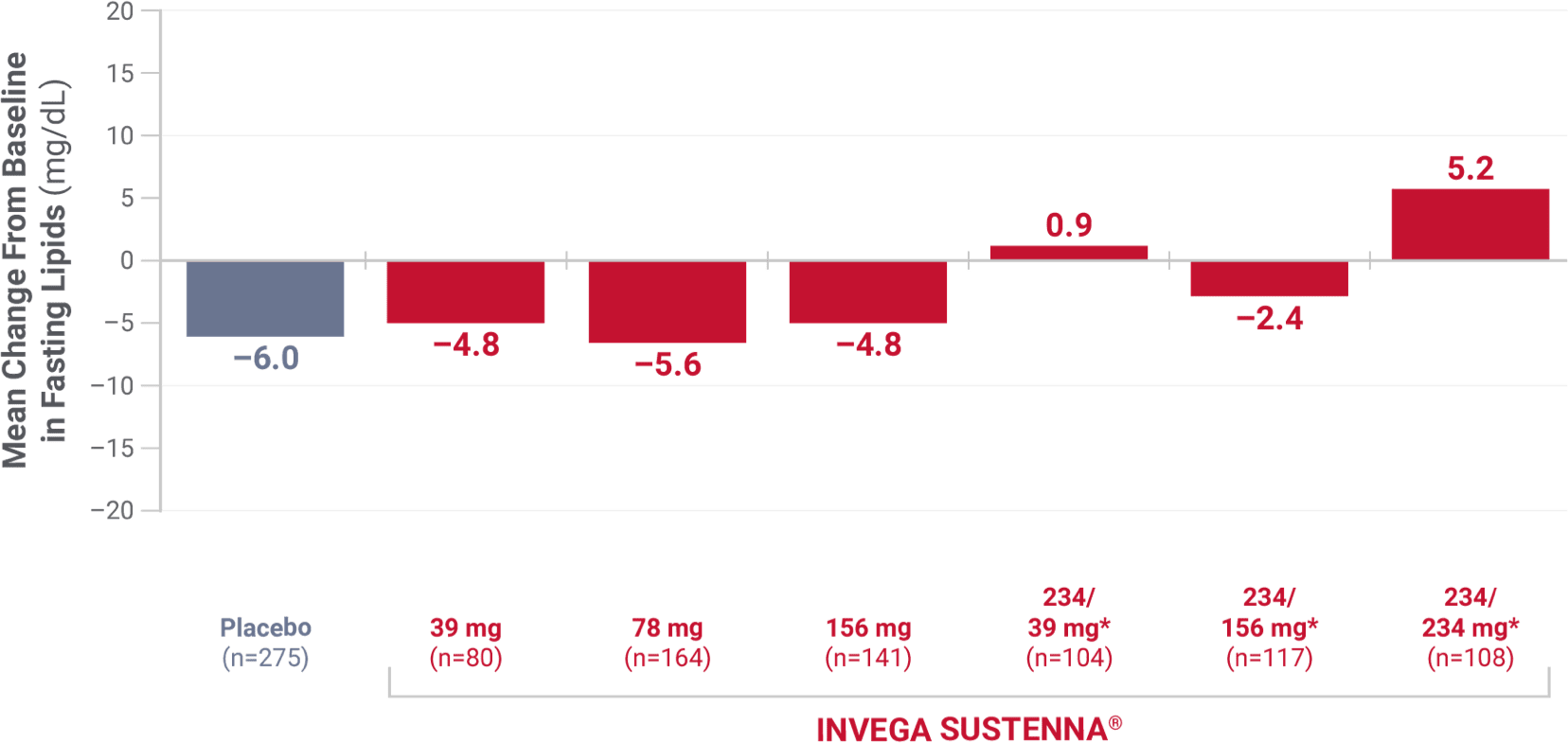
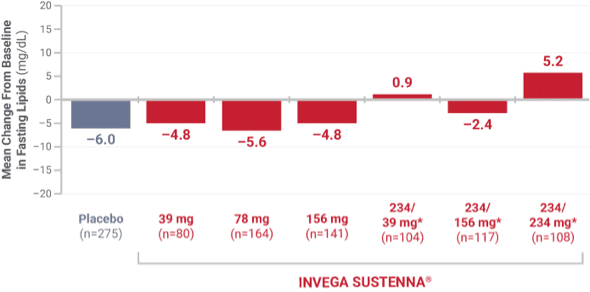
High-density lipoprotein (HDL)
From 4 placebo-controlled, 9- to 13-week, fixed-dose studies
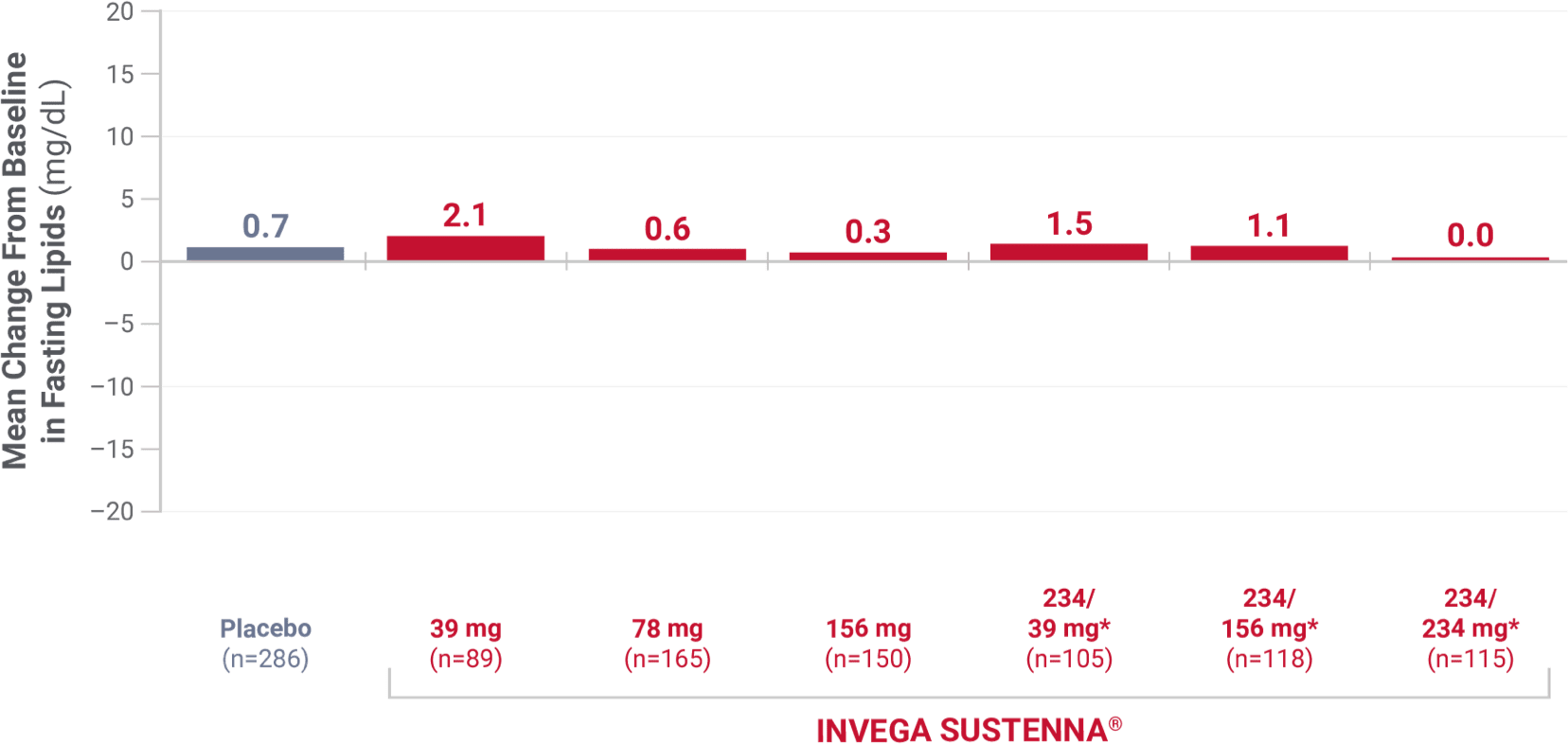
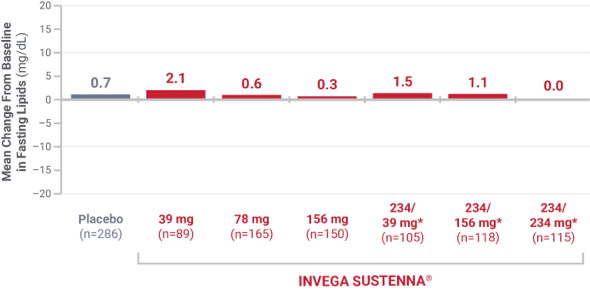
Triglycerides
From placebo-controlled, 9- to 13-week, fixed-dose studies
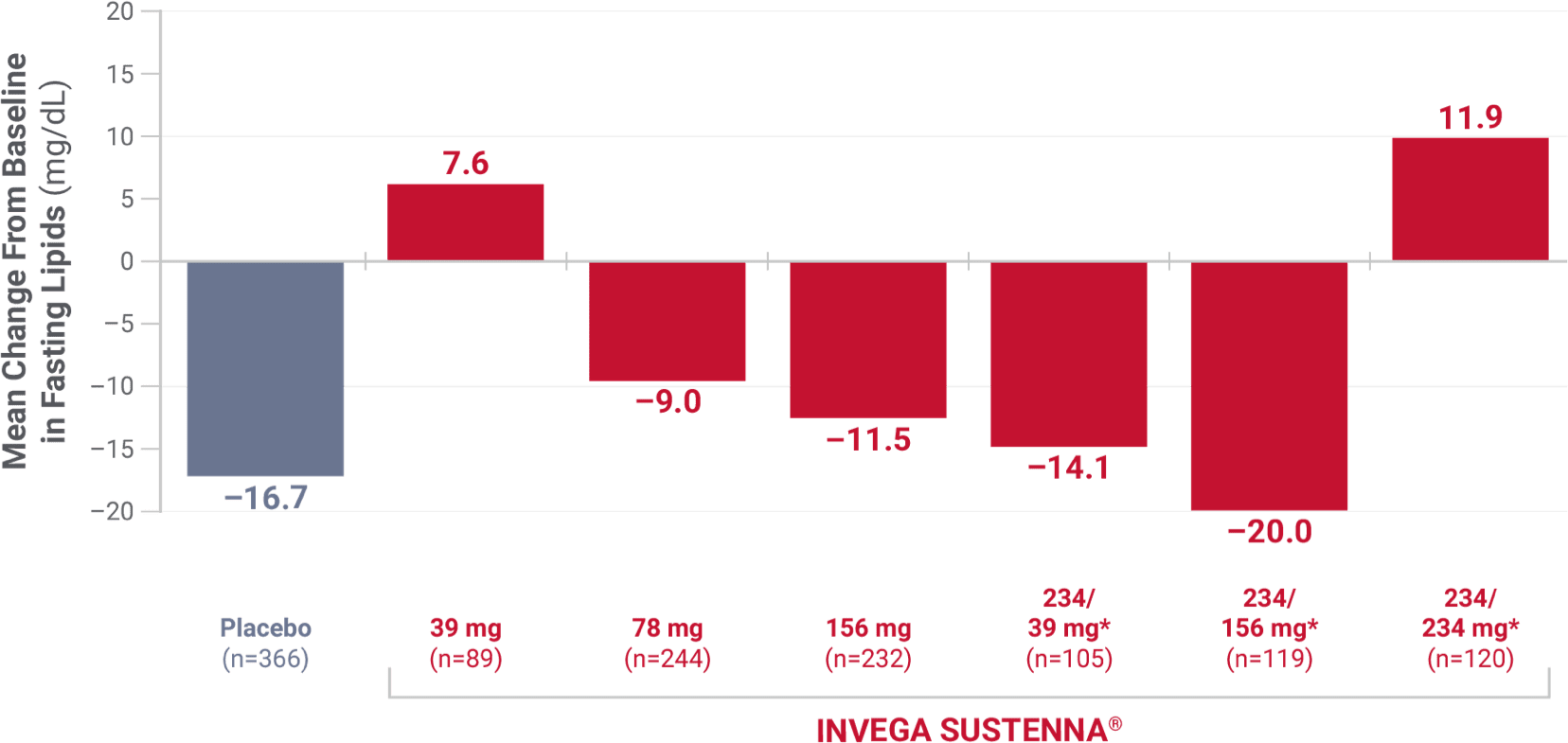
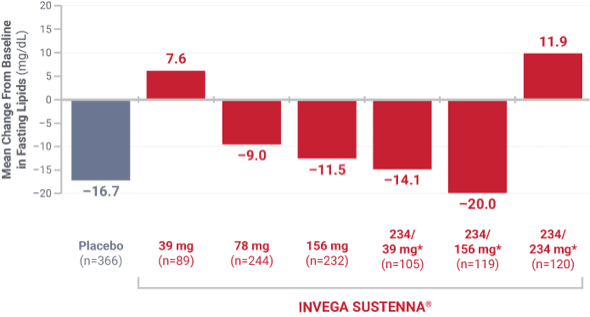
DYSLIPIDEMIA: Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics.1
*Initial deltoid injection of 234 mg followed by 39 mg, 156 mg, or 234 mg every 4 weeks by deltoid or gluteal injection. Other dose groups (39 mg, 78 mg, and 156 mg) are from studies involving only gluteal injection.1
Reference: 1. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.