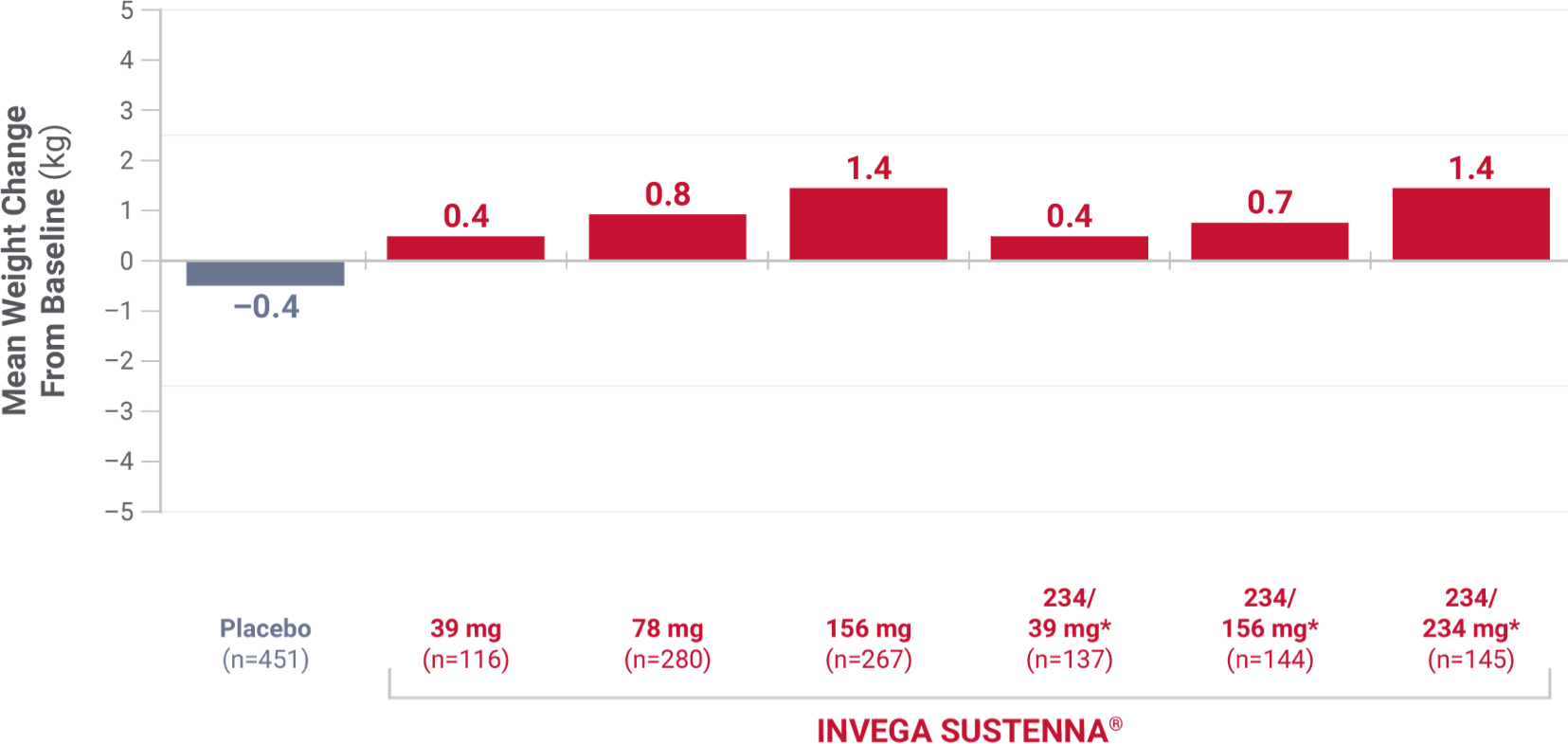
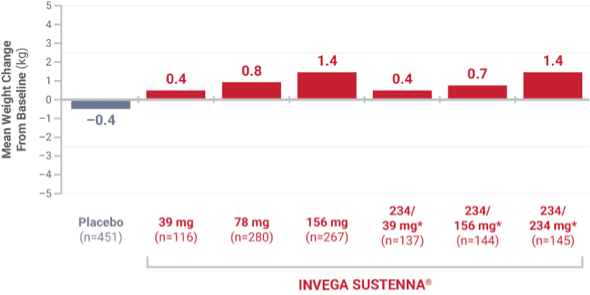
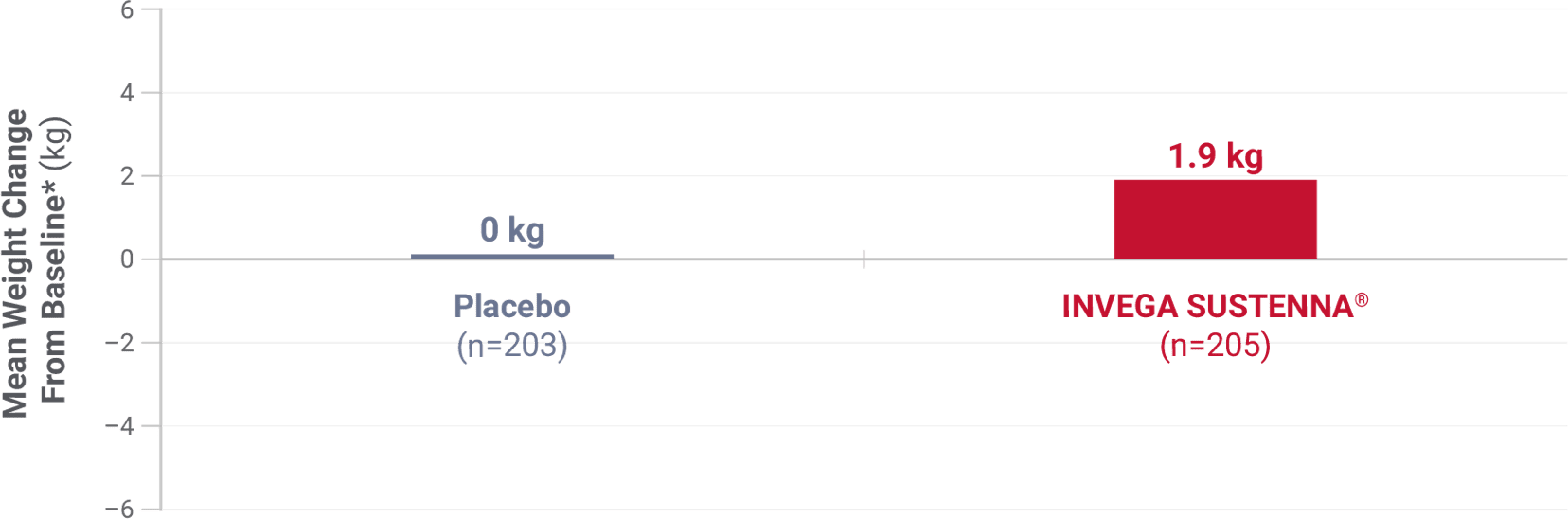
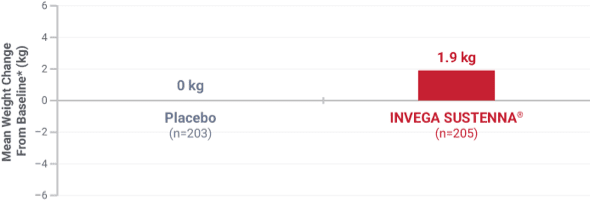
Weight change was evaluated across short-term and long-term pivotal trials
Weight change from short-term studies
Results from 4 placebo-controlled (one 9-week and three 13-week), fixed-dose studies of adult patients experiencing an acute exacerbation of schizophrenia.1