FOR US HEALTHCARE PROFESSIONALS ONLY
INVEGA SUSTENNA® was extensively evaluated in pivotal studies vs placebo
IN A LONGER-TERM STUDY
INVEGA SUSTENNA® demonstrated significantly longer time to relapse vs placebo (P<0.0001)1,2
90% of patients remained relapse-free at the interim analysis of a longer-term study1
90% of patients remained relapse-free at the interim analysis of a longer-term study1
- Due to the significant efficacy of INVEGA SUSTENNA®, the study was terminated early at the preplanned interim analysis by an Independent Data Monitoring Committee1
- The most common adverse reactions from the 5 pivotal trials (incidence ≥5% and occurring at least twice the rate of placebo) were injection-site reactions, somnolence/sedation, dizziness, akathisia, and extrapyramidal disorder1
Relapse criteria
In the longer-term study, relapse was defined as experiencing ≥1 of the following1,2:
- Psychiatric hospitalization
- ≥25% increase in PANSS total score*
- Increase in individual PANSS item scores†
- Deliberate self-injury, violent behavior
- Suicidal or homicidal ideation
This study was not powered to draw conclusions for individual reasons for relapse.
Indicated for the treatment of schizophrenia in adults.
The full constellation of symptoms and the relevant diagnostic criteria should be consulted and are available in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5-TR®, or current version), where applicable.
IN A LONGER-TERM STUDY
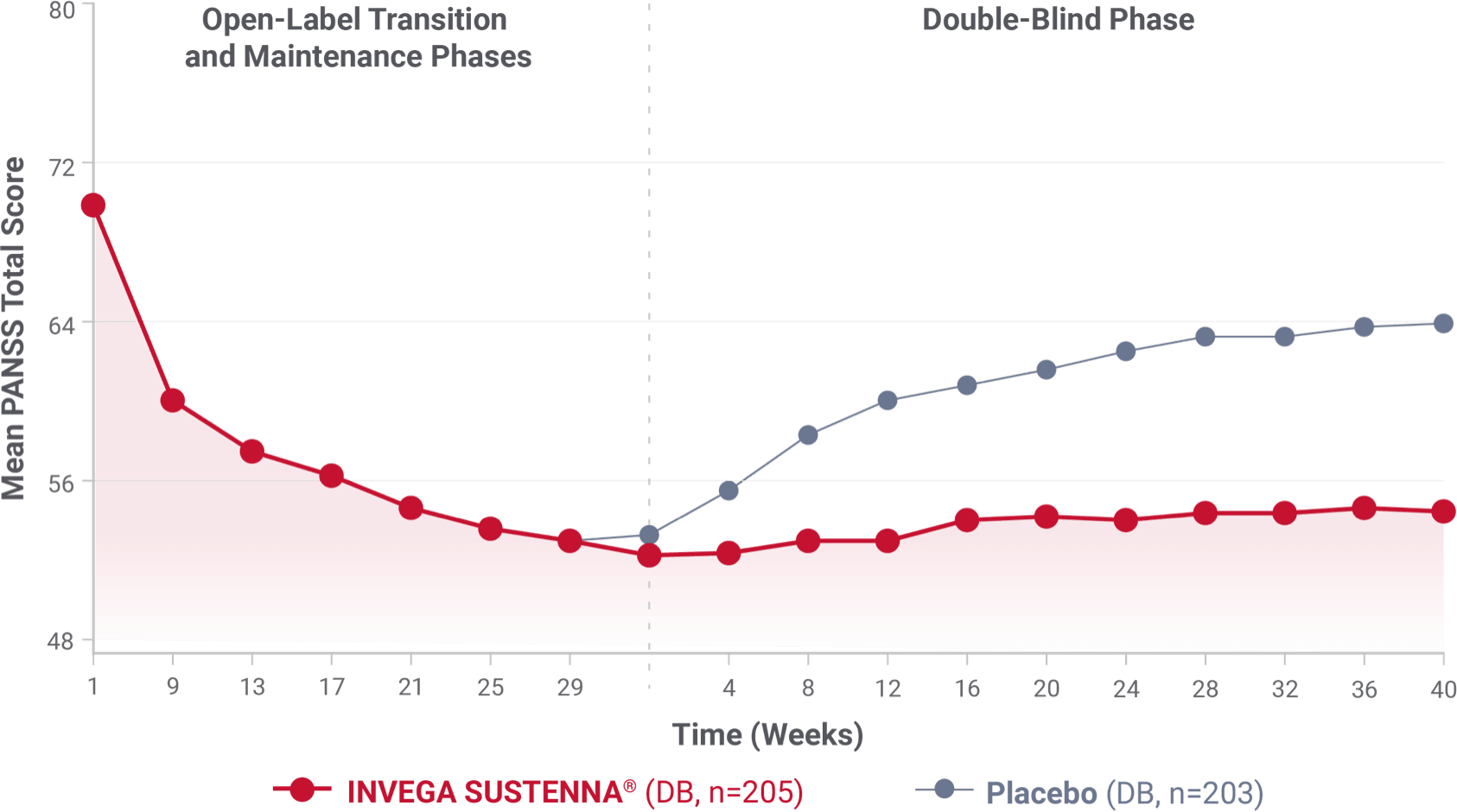
The mean PANSS total score remained stable for patients on INVEGA SUSTENNA® while significantly worsening for patients on placebo2*
Secondary endpoint: change in mean PANSS total score over time2
Long-term study design (abbreviated)2
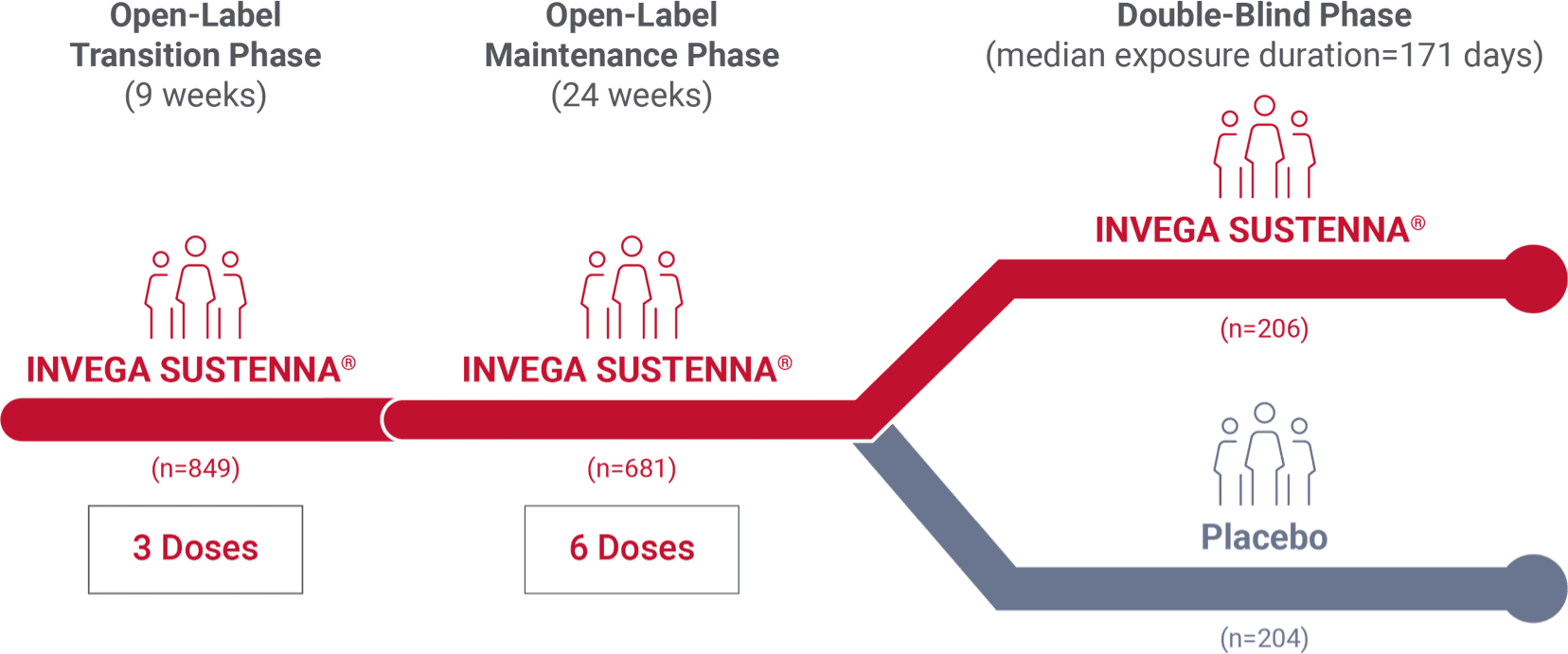
- Results from the longer-term, placebo-controlled study of adult patients with schizophrenia. Patients were treated for 33 weeks during an open-label stabilization phase with INVEGA SUSTENNA® (39 mg, 78 mg, or 156 mg every 4 weeks after initialization)1,2
- Patients were then randomized to this same dose or to placebo in a variable-length, double-blind phase. A preplanned interim analysis was conducted after 68 relapse events1,2
Study notes
- Median duration of total exposure in the INVEGA SUSTENNA® arm was 13 months2
- Due to significant efficacy of INVEGA SUSTENNA®, the study was stopped early after the preplanned interim analysis by an Independent Data Monitoring Committee1,2
- The median time to relapse in the placebo arm of the double-blind phase was 163 days2
- 36% of adults were diagnosed within 5 years, with a mean age of 33 years (the mean age of all the patients in the study was 39 years [n=408])1,2
IN A SHORT-TERM STUDY
INVEGA SUSTENNA® demonstrated significant symptom improvement in PANSS total scores1,3
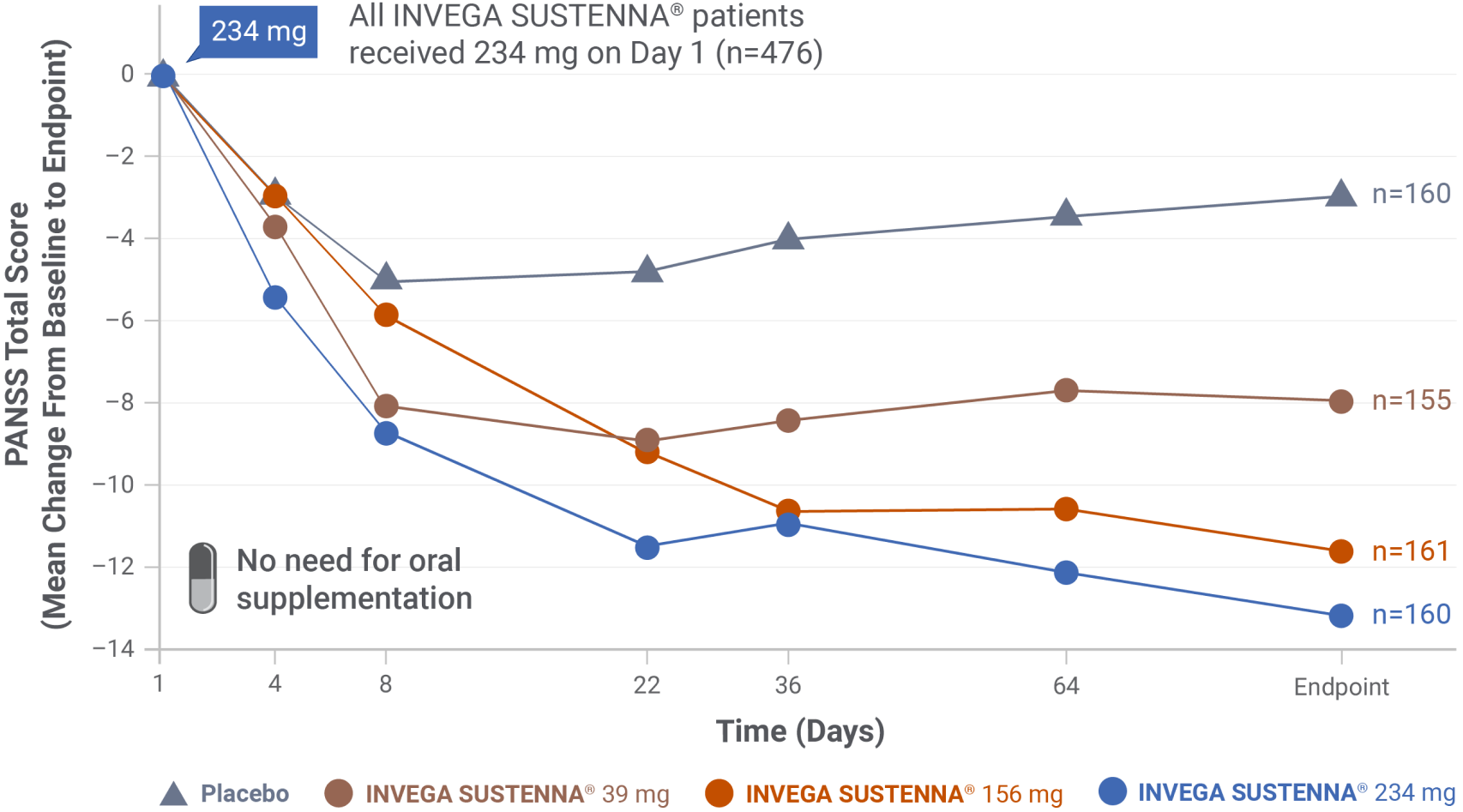
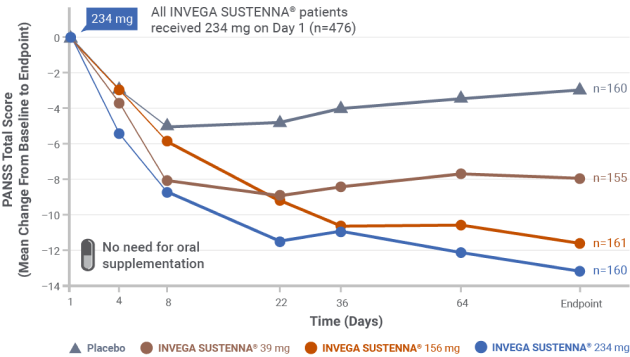
- Doses were administered on Day 1 (234 mg) and Days 8, 36, and 64 (per dosage strength in study design)
- NOTE: Two deltoid intramuscular injections of 234 mg (on Day 1) and 156 mg (on Day 8) are the recommended initiation doses for INVEGA SUSTENNA®
PANSS=Positive and Negative Syndrome Scale.
IN A SHORT-TERM STUDY
Demonstrated change in PANSS subscales was evaluated3
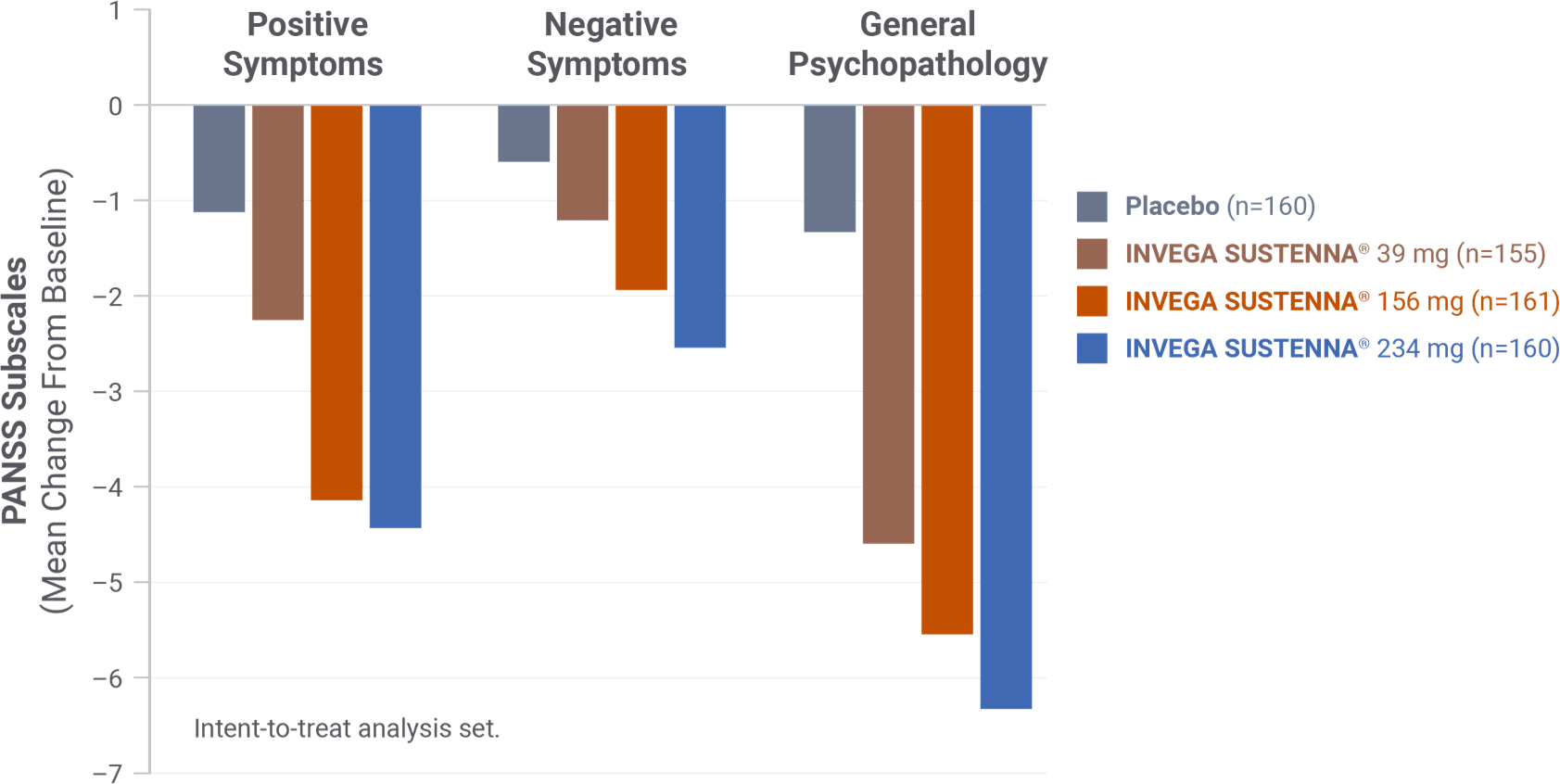
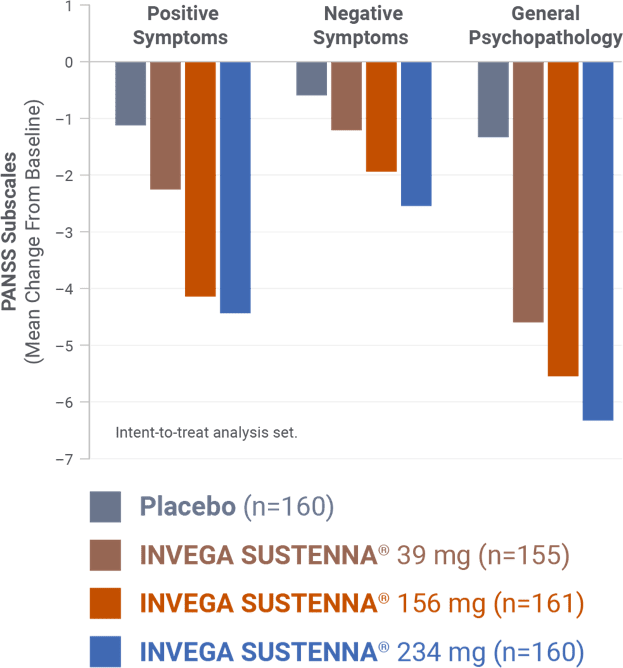
INVEGA SUSTENNA® demonstrated significant symptom improvement in PANSS total scores1,3
- The study was not powered to draw conclusions for individual factors of the PANSS
PANSS=Positive and Negative Syndrome Scale.
PANSS subscales1,4
The Positive and Negative Syndrome Scale (PANSS) is a 30-item scale that measures positive and negative symptoms of schizophrenia, as well as general psychopathology.1,4 The PANSS subscales can be used to assess symptom changes.4
- 7-item positive scale, measuring symptoms added to normal mental status4
- 7-item negative scale, assessing features absent from normal mental status4
- 16-item general psychopathology scale, measuring the overall severity of schizophrenia4
POSITIVE SCALE
- Delusions
- Conceptual disorganization
- Hallucinatory behavior
- Excitement
- Grandiosity
- Suspiciousness
- Hostility
NEGATIVE SCALE
- Blunted affect
- Emotional withdrawal
- Poor rapport
- Passive-apathetic social withdrawal
- Difficulty in abstract thinking
- Lack of spontaneity and flow of conversation
- Stereotyped thinking
General Psychopathology Scale
- Somatic concern
- Anxiety
- Guilt feelings
- Tension
- Mannerisms and posturing
- Depression
- Motor retardation
- Uncooperativeness
- Unusual thought content
- Disorientation
- Poor attention
- Lack of judgment and insight
- Disturbance of volition
- Poor impulse control
- Preoccupation
- Active social avoidance
Short-term (13-week) study design
Results from a double-blind, randomized, placebo-controlled, fixed-dose, 13-week study of adult patients experiencing an acute exacerbation of schizophrenia. Patients were randomized to receive placebo or a 234-mg deltoid injection dose on Day 1, followed by a 30 mg, 156 mg, or 234 mg dose in either the deltoid or gluteal muscle on Day 8, and once monthly thereafter.1,3
Back to Top