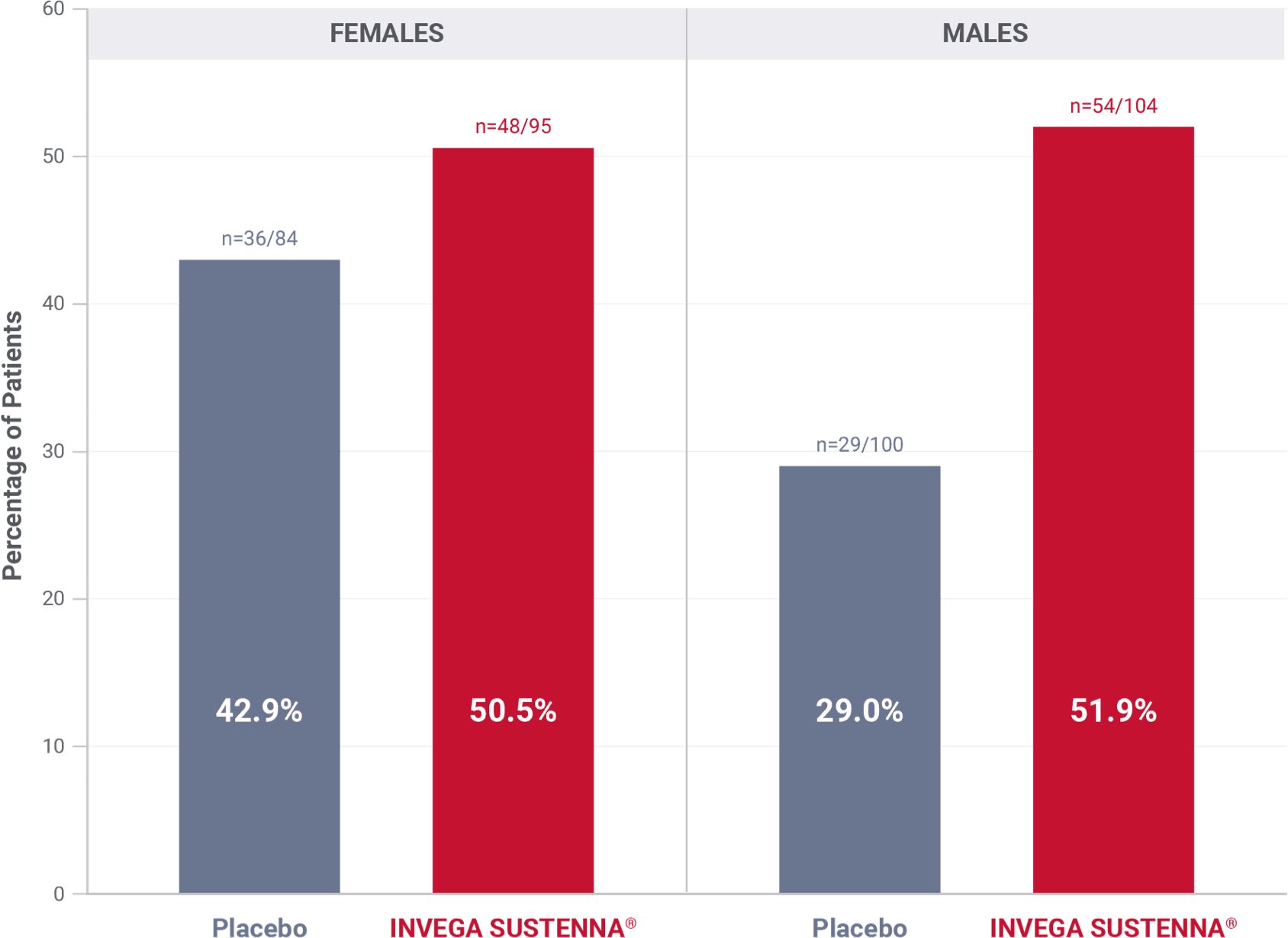
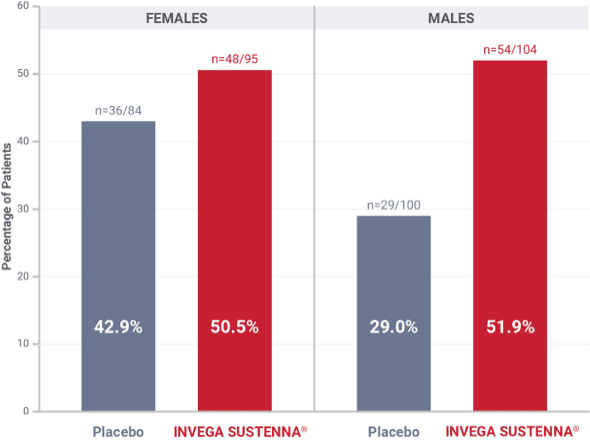
Prolactin elevations in the double-blind phase of the long-term maintenance trial in schizophrenia1*
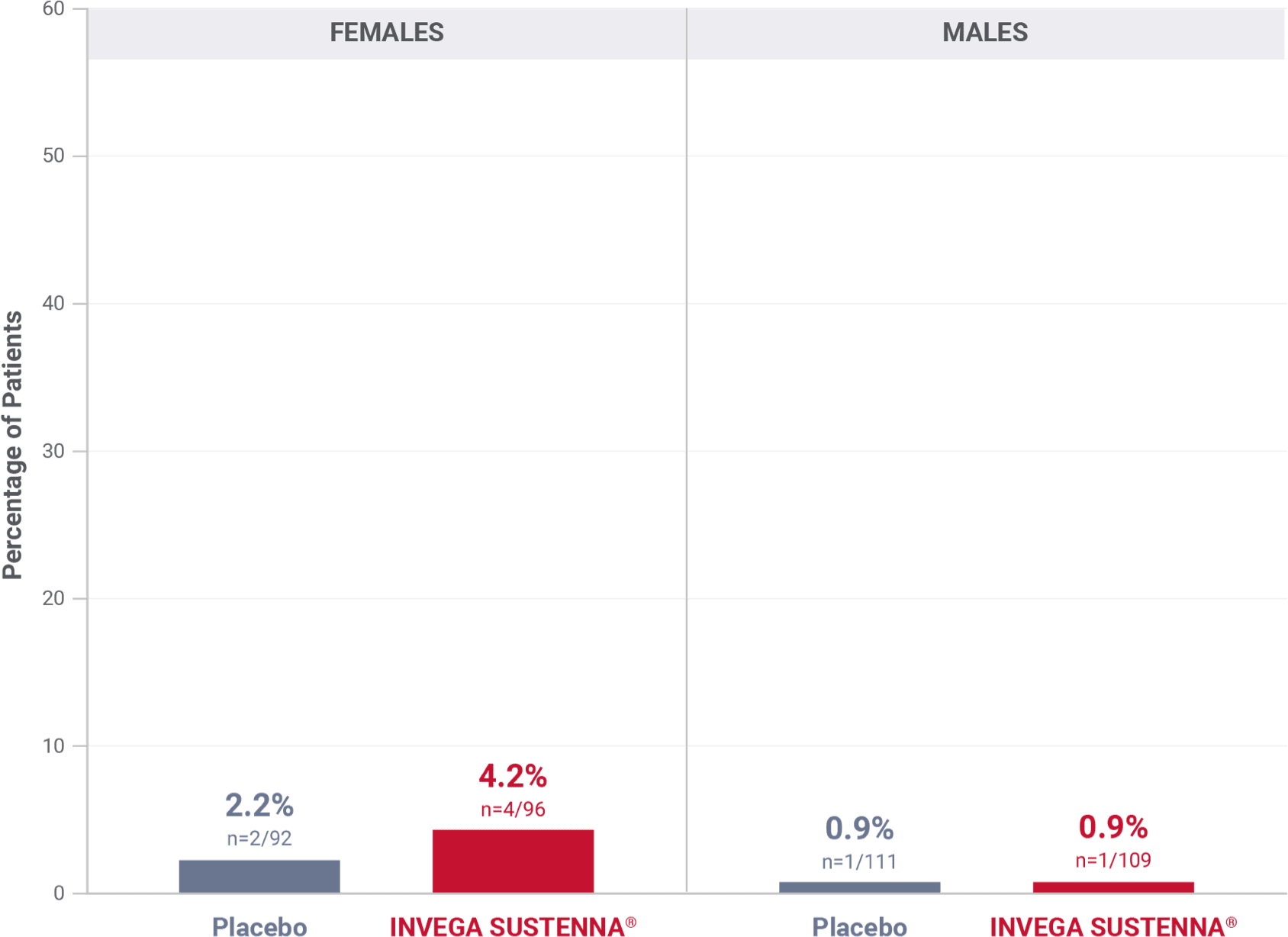
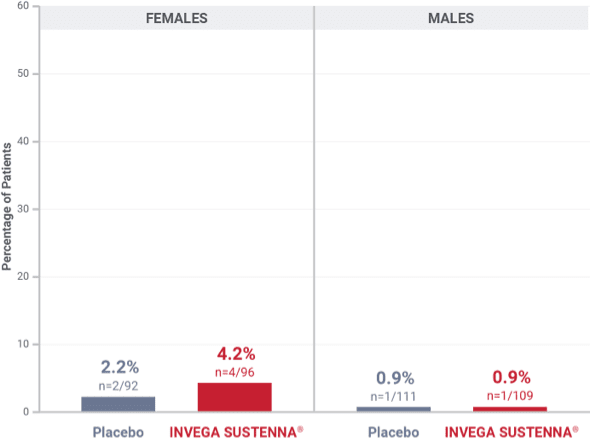
Percentage of patients with potentially prolactin-related adverse reactions in the double-blind phase of the long-term maintenance trial in schizophrenia1†
*Elevations of prolactin to above the reference range (>18 ng/mL in males and >30 ng/mL in females) relative to open-label baseline at any time during the double-blind phase.
†Prolactin adverse reaction reports among all patients in the double-blind phase, regardless of baseline prolactin levels.
‡In the INVEGA SUSTENNA® group, 2 females reported amenorrhea, 1 reported galactorrhea, and 1 reported irregular menstruation, while in the placebo group, 1 female reported amenorrhea and 1 reported breast pain.
§In the INVEGA SUSTENNA® group, 1 male reported erectile dysfunction, and in the placebo group, 1 male reported gynecomastia.
Reference: 1. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.