FOR US HEALTHCARE PROFESSIONALS ONLY
INVEGA HAFYERA® was evaluated in a 1-year noninferiority study and a 2-year, open-label extension study1-3
IN A 1-YEAR NONINFERIORITY STUDY
INVEGA HAFYERA® delayed relapse as effectively as INVEGA TRINZA®1
At 1 Year
92.5%
of patients taking INVEGA HAFYERA®
VS
95%
of patients taking INVEGA TRINZA®
remained relapse-free
Results of a 12-month, randomized, double-blind, noninferiority trial. The primary efficacy variable was time to first relapse in the double-blind study.1,2
A total of 702 stabilized patients were randomized in a 2:1 ratio to receive INVEGA HAFYERA® (n=478) or INVEGA TRINZA® (n=224) over the 12-month, double-blind study.2
Safety and adverse reactions1
- In the double-blind phase, 1.3% of patients in the INVEGA HAFYERA® group and 0.4% of patients in the INVEGA TRINZA® group discontinued due to adverse events
- The most common adverse reactions (≥5%) in the INVEGA HAFYERA® group were upper respiratory tract infection (12%), injection-site reaction (11%), weight increased (9%), headache (7%), and extrapyramidal symptoms* (7%)
- The most common adverse reactions (≥5%) in the INVEGA TRINZA® group were upper respiratory tract infection (13%), weight increased (8%), injection-site reaction (5%), extrapyramidal symptoms* (5%), and headache (5%)
Extrapyramidal symptoms include: blepharospasms, bradykinesia, drooling, dyskinesia, dystonia, hypokinesia, musculoskeletal stiffness, muscle rigidity, muscle spasms, oculogyric crisis, parkinsonism, parkinsonism rest tremor, reduced facial expression, tardive dyskinesia.
IN A 2-YEAR OPEN-LABEL EXTENSION STUDY
Patients who were relapse-free for 1 year in the pivotal study were eligible to enter an open-label extension study for 2 years3
178 patients who were relapse-free on INVEGA HAFYERA® (n=121) or INVEGA TRINZA® (n=57) in the double-blind phase chose to continue treatment with INVEGA HAFYERA® in the open-label extension.3
2-year, real-world, open-label extension safety and tolerability study3*

96.1%
of patients who entered the study
remained relapse-free on INVEGA HAFYERA®3
7 out of 178 patients who entered the open-label phase relapsed*
87% of patients (154/178) completed the 2-year, open-label study†
7 out of 178 patients who entered the open-label phase relapsed*
87% of patients (154/178) completed the 2-year, open-label study†
Safety and adverse reactions
The most common (≥5%) treatment-emergent adverse events were headache (13.5%), blood prolactin increased (10.7%), hyperprolactinemia (7.3%), diarrhea (6.2%), weight increased (5.1%), and nasopharyngitis (5.1%).3‡
The open-label phase of the study was conducted in 6 countries, excluding the United States.
Intent-to-treat population (n=178).
In the open-label extension design, investigators were not blinded to prolactin laboratory results. Comparisons between double-blind studies and OLE studies should not be made.
Relapse criteria1-3
Relapse was defined as any of the following:
- Psychiatric hospitalization
- Increase of ≥25% in total PANSS score from randomization for 2 consecutive assessments (if baseline score was >40) (pivotal study only)
- 10-point increase in total PANSS score for 2 consecutive assessments (if baseline score was ≤40) (pivotal study only)
- Deliberate self-injury, violent behavior, or suicidal/homicidal ideation
- Score of ≥5 (if the maximum baseline score was ≤3) or ≥6 (if the maximum baseline score was 4) on 2 consecutive assessments of the specific PANSS items
- Emergency department/room/ward visit due to a worsening of the subject’s symptoms of schizophrenia (OLE only)
OLE=open-label extension; PANSS=Positive and Negative Syndrome Scale.
Patients who completed the open-label extension study maintained treatment for a total of 3 years1-3
Mean PANSS total scores2,3

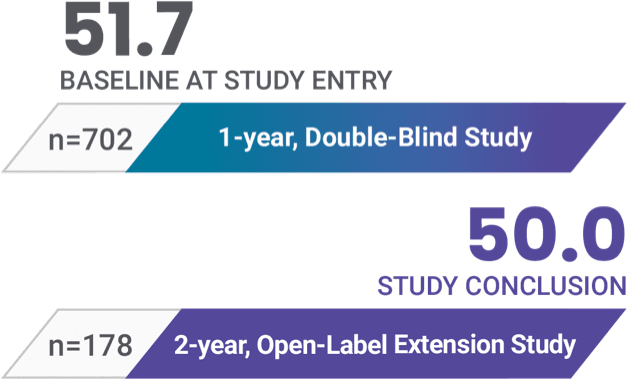
178 patients who were relapse-free for 1 year on INVEGA HAFYERA® (n=121) or INVEGA TRINZA® (n=57) in the double-blind study chose to continue treatment with INVEGA HAFYERA® in the open-label extension.3
At study entry, all patients were required to have a PANSS total score ≤70.2
PANSS=Positive and Negative Syndrome Scale.
Noninferiority and open-label extension study design
Of the 1,036 participants who entered the initial screening phase, 702 continued on to the 12-month, randomized, double-blind phase of the noninferiority trial.1-3
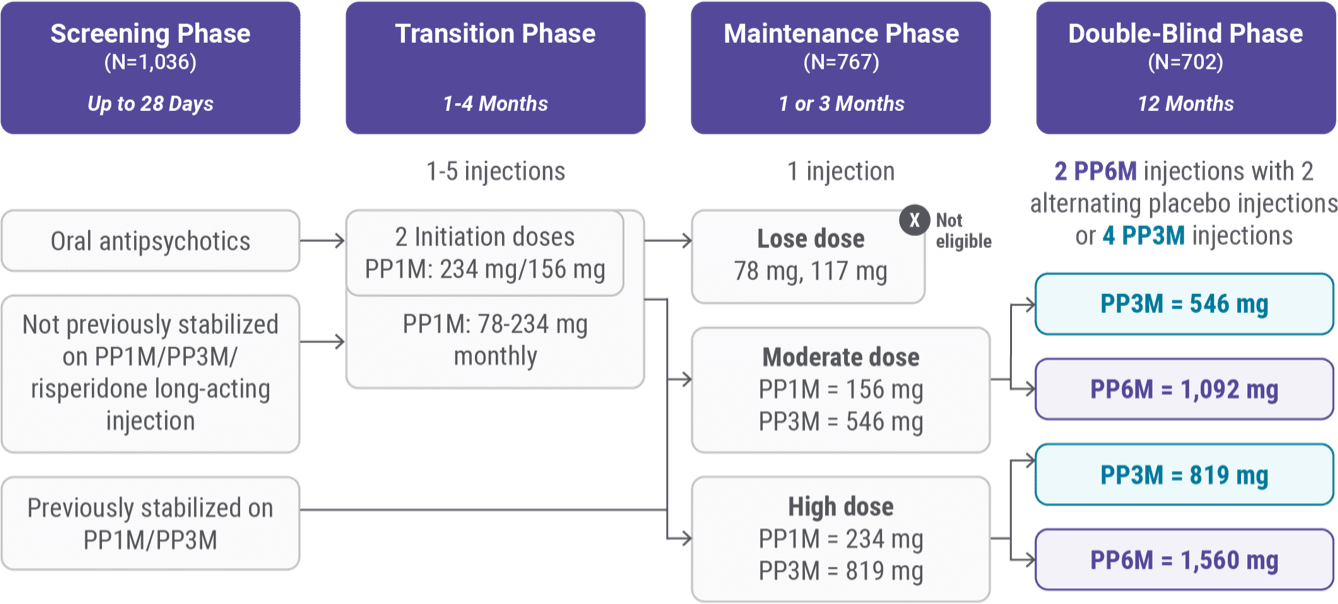
Screening phase
1,036 patients entered the initial screening phase:
- Patients on INVEGA SUSTENNA®
- Patients on INVEGA TRINZA®
- Patients on injectable risperidone
- Patients on oral antipsychotics except clozapine
Transition phase
Patients who had not been stabilized on INVEGA SUSTENNA® or INVEGA TRINZA® entered a transition phase to be stabilized with 1 to 5 injections of INVEGA SUSTENNA®.
- Patients on oral antipsychotics were given 2 initiation doses (234 mg followed by 156 mg one week later), with both initiation doses administered in the deltoid muscle, and transitioned to 3 maintenance doses of 78 mg to 234 mg
- Patients on 50 mg of injectable risperidone were transitioned to 156 mg of INVEGA SUSTENNA® followed by 3 maintenance doses of 78 mg to 234 mg
- Stability was defined as at least 3 months of maintenance injections, with the last 2 doses being the same strength
Maintenance phase
In the maintenance phase, 767 patients received 1 injection of INVEGA SUSTENNA® or INVEGA TRINZA® through either straightforward progression or established conversion.
Patients on doses of INVEGA SUSTENNA® (78 mg and 117 mg) in the transition phase did not proceed to the maintenance phase.
High dose:
- 234 mg of INVEGA SUSTENNA®
- 819 mg of INVEGA TRINZA®
Moderate dose:
- 156 mg of INVEGA SUSTENNA®
- 546 mg of INVEGA TRINZA®
Double-blind phase
In the double-blind phase, 702 patients who met the criteria for clinical stability were randomized in a 2:1 ratio to receive an injection every 3 months over a 12-month period.
- The INVEGA HAFYERA® group (n=478) received 2 injections of INVEGA HAFYERA® with 2 alternating placebo injections
- The INVEGA TRINZA® (n=224) group received 4 injections of INVEGA TRINZA®
PP1M=paliperidone palmitate 1-month(INVEGA SUSTENNA®); PP3M=paliperidone palmitate3-month (INVEGA TRINZA®); PP6M=paliperidone palmitate6-month (INVEGA HAFYERA®).
Patients who did not relapse in the noninferiority study could opt to enroll in theopen-label extension. Of the 178 patients enrolled, 154 completed the study.
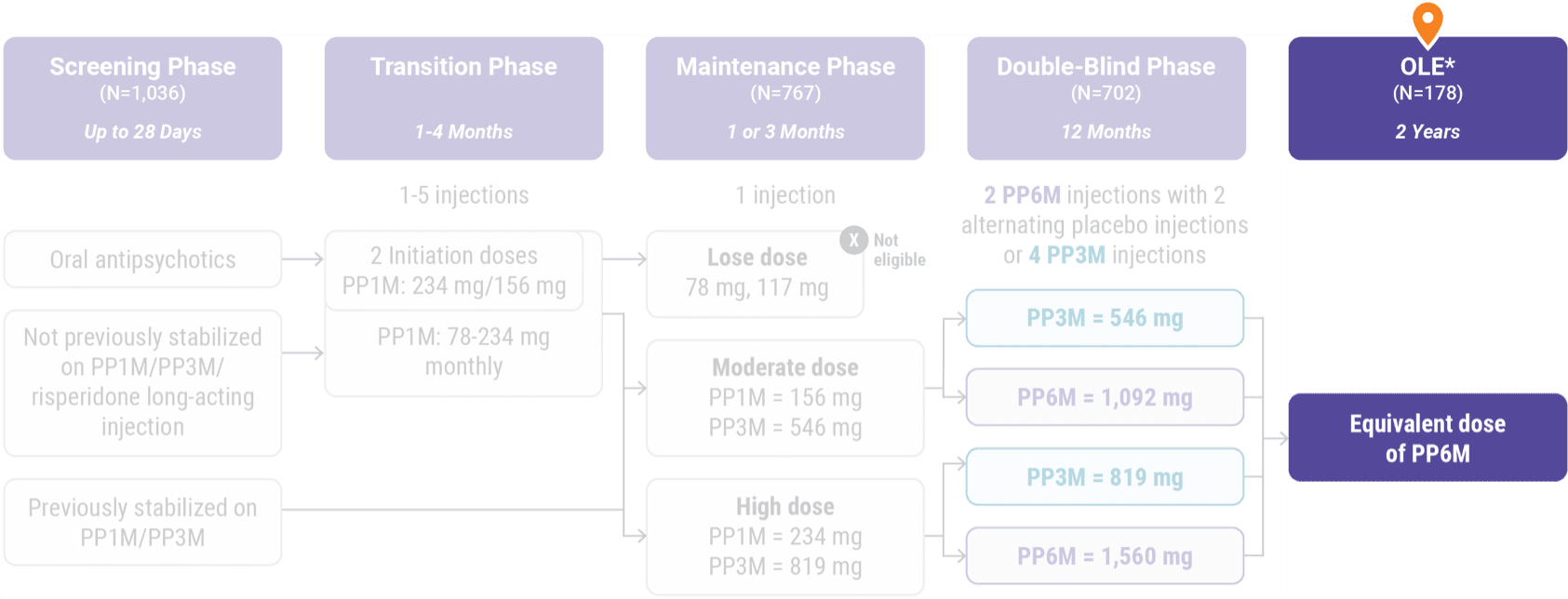
Open-label extension
1,036 patients entered the initial screening phase:
- Open-label enrollment included 121 patients who received INVEGA HAFYERA® and 57 patients who received INVEGA TRINZA® in the double-blind phase of the noninferiority study and did not relapse
- Enrolled subjects received up to 4 injections of INVEGA HAFYERA® at baseline, 6-month, 12-month, and 18-month visits. The objective was to evaluate the safety and tolerability of INVEGA HAFYERA®
Study criteria
Pivotal study key inclusion criteria2
- Age: 18 to 70 years
- Diagnosed with schizophrenia (per DSM-5®) for at least 6 months before screening
- Receiving treatment with INVEGA SUSTENNA®, INVEGA TRINZA®, injectable risperidone, or any oral antipsychotic
- Total PANSS scores of <70 points at screening and at randomization
Open-label extension key inclusion criteria3
- Completed the double-blind phase of pivotal study without relapse and willing to continue treatment with INVEGA HAFYERA®
Key exclusion criteria2
- Active primary DSM-5® diagnosis other than schizophrenia
- Receiving any form of involuntary treatment
- Suicide attempt within 12 months before screening or imminent risk of suicide or violent behavior
- DSM-5® diagnosis of moderate or severe substance use disorder (except for nicotine and caffeine) within 6 months of screening
- History of NMS or TD
- Unstable medical conditions
- History of unresponsiveness or intolerance to paliperidone/risperidone
DSM-5=Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; NMS=neuroleptic malignant syndrome; PANSS=Positive and Negative Syndrome Scale; TD=tardive dyskinesia.
Back to Top