FOR US HEALTHCARE PROFESSIONALS ONLY
INVEGA TRINZA® prolactin data
Hyperprolactinemia: As with other drugs that suppress dopamine D2 receptors, INVEGA TRINZA® can elevate prolactin levels and the elevation persists during chronic administration. Paliperidone has a prolactin-elevating effect similar to risperidone, which is associated with higher levels of prolactin elevation than other antipsychotic agents.1
Elevated prolactin levels in patients taking INVEGA TRINZA®
- Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotrophin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male patients1
- Counsel patients on signs and symptoms of hyperprolactinemia that may be associated with chronic use of INVEGA TRINZA®. Advise them to seek medical attention if they experience any of the following: amenorrhea or galactorrhea in females; erectile dysfunction or gynecomastia in males1
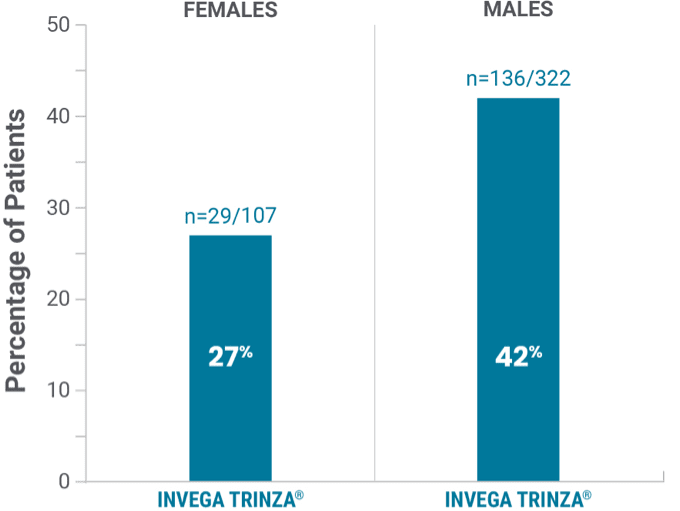
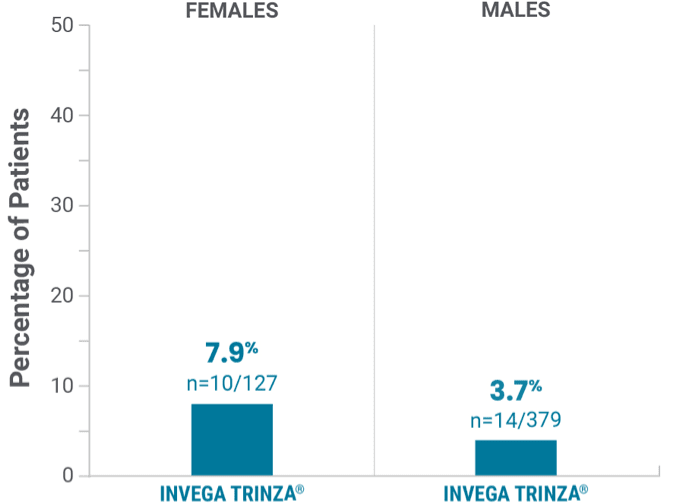
Observed in the double-blind phase
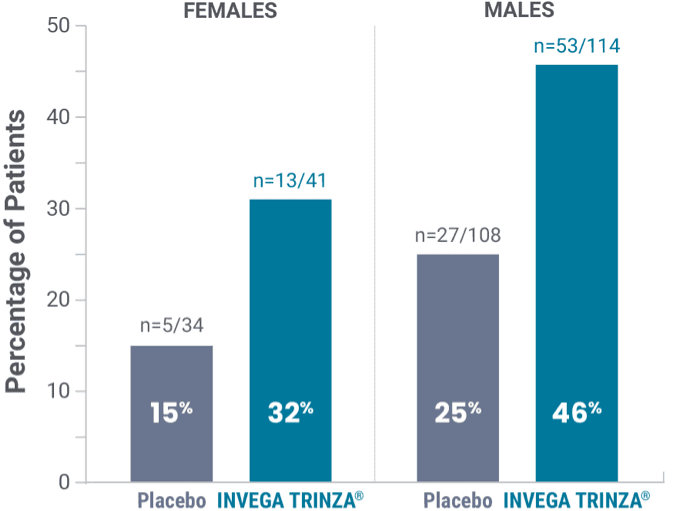
Prolactin elevations1,2*
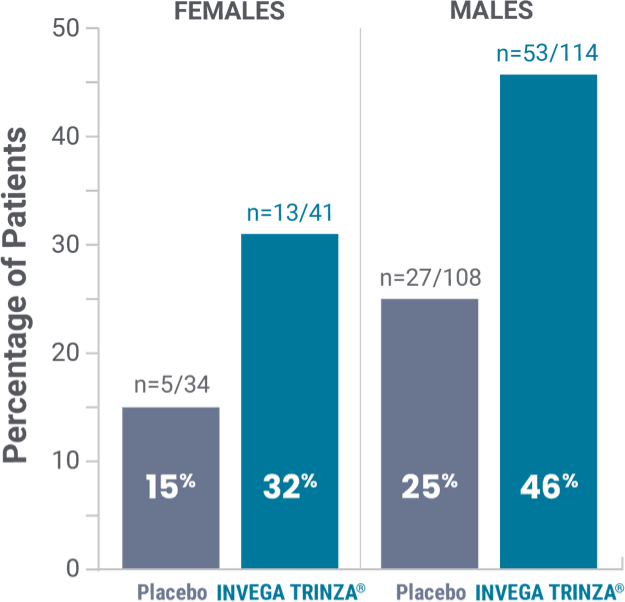
Potentially prolactin-related adverse reactions1,3†
*Elevations of prolactin to above the reference range (>13.13 ng/mL in males and >26.72 ng/mL in females) relative to open-label baseline at any time during the double-blind phase.
†Prolactin adverse reaction reports among all patients in the double-blind phase, regardless of baseline prolactin levels.
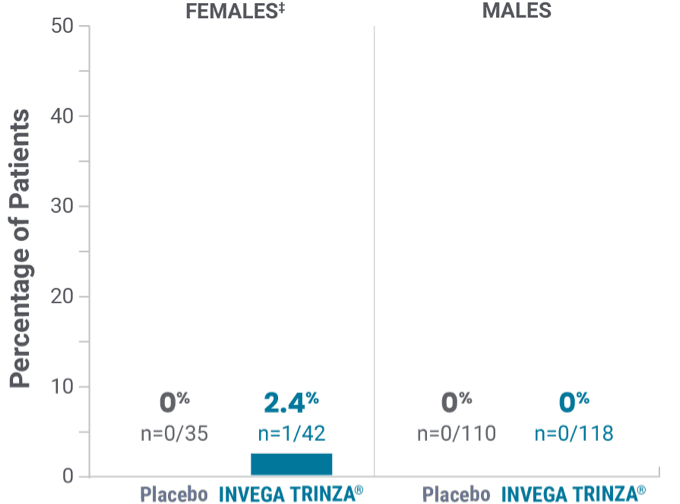
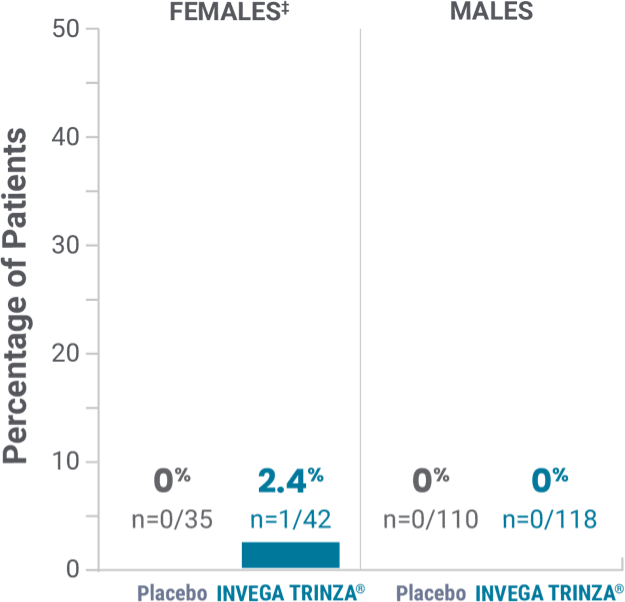
‡One female on INVEGA TRINZA® reported amenorrhea.
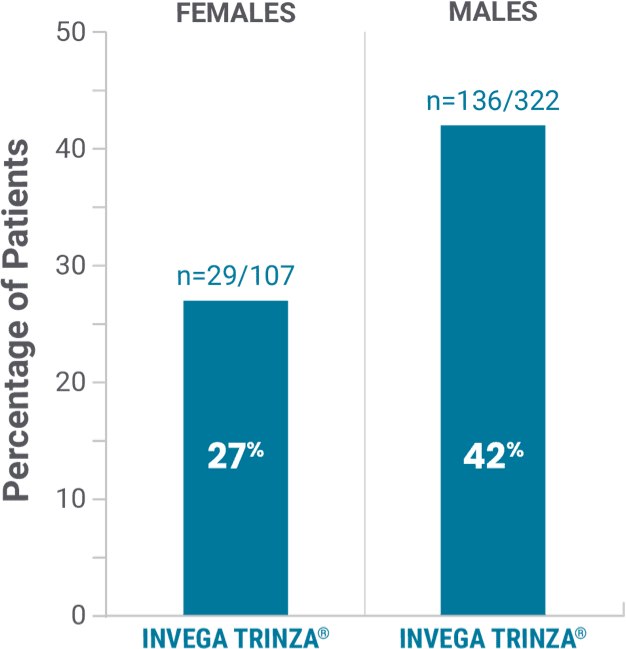
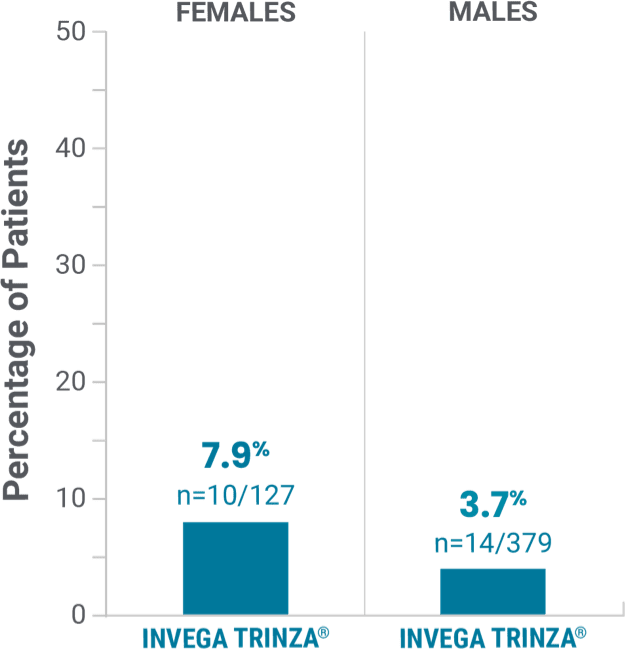
Observed in the open-label phase*†
Prolactin elevations1,2
Potentially prolactin-related adverse reactions1,3†
| Most commonly observed potentially prolactin-related adverse reactions (≥3%) | ||
|---|---|---|
| FEMALES | MALES | |
| Amenorrhea | 4.7% (6/127) | Not applicable |
| Galactorrhea | 3.1% (4/127) | 0% (0/379) |
*Elevations of prolactin to above the reference range (>13.13 ng/mL in males and >26.72 ng/mL in females) relative to open-label baseline at any time during the double-blind phase.
†During the open-label phase, subjects received several doses of INVEGA SUSTENNA® followed by a single dose of INVEGA TRINZA®.
‡Prolactin adverse reaction reports among all patients in the open-label phase, regardless of baseline prolactin levels.
Back to Top