FOR US HEALTHCARE PROFESSIONALS ONLY
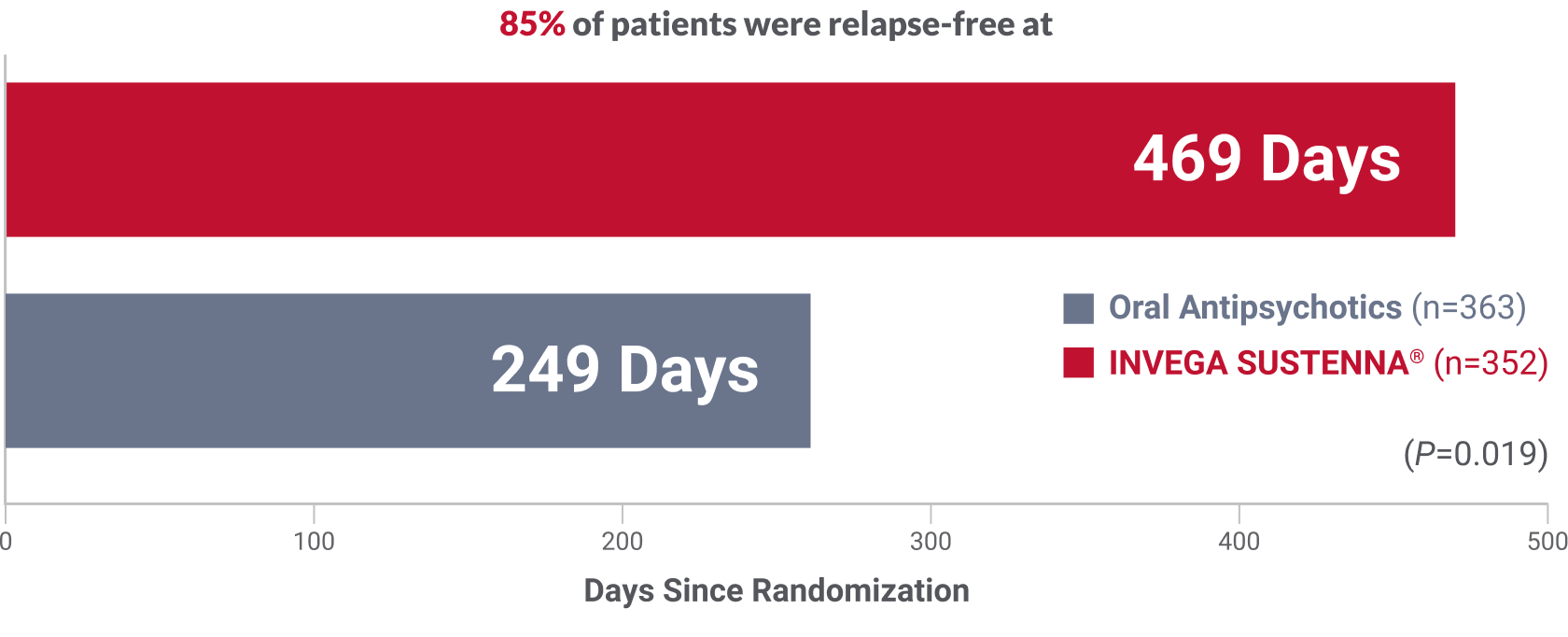
INVEGA SUSTENNA® was evaluated in a 24-month international† study vs a group of 6 common oral antipsychotics1
Over 7 months’ difference in time to relapse
compared to a group of oral antipsychotics
Oral antipsychotics received by patients:
aripiprazole, haloperidol, olanzapine, paliperidone ER, quetiapine, risperidone
Primary endpoint: Significantly longer time to relapse in patients receiving INVEGA SUSTENNA® vs oral antipsychotics (P=0.019; HR=1.5, 95% CI=1.1-2.2)1‡
Secondary endpoint: Significantly fewer patients relapsed in the INVEGA SUSTENNA® vs oral antipsychotics arm§ (14.8% and 20.9%, respectively; P=0.032)1
- The study was not powered to compare the efficacy of INVEGA SUSTENNA® with that of individual oral antipsychotics
- The percentage of patients who discontinued due to adverse events was 4.0% in the INVEGA SUSTENNA® group and 3.0% in the oral antipsychotic group1
- The 5 most common treatment-emergent adverse events in the INVEGA SUSTENNA® group were weight increase (15.9%), headache (11.1%), insomnia (9.7%), schizophrenia (8.2%), and nasopharyngitis (7.1%)1
- The 5 most common treatment-emergent adverse events in the oral antipsychotics group were weight increase (17.4%), schizophrenia (9.6%), headache (8.5%), insomnia (8.0%), and suicidal ideation (5.5%)1
- 2 patients discontinued treatment due to injection-related issues2
Relapse criteria1
Relapse was defined as any of the following:
- Psychiatric hospitalization
- Increase in level of psychiatric care (eg, significant crisis intervention to avert hospitalization, clinically notable increases in frequency or intensity of patient contact required to maintain outpatient status) and increase of 25% from baseline in PANSS total score (or an increase of 10 points if baseline score ≤40)
- Deliberate self-injury
- Clinically significant suicidal or homicidal ideation
- Violent behavior resulting in clinically significant injury to another person or property damage
- Substantial clinical deterioration: 6 (much worse) or 7 (very much worse) on the CGI change scale
- Required antipsychotic dose exceeds maximum approved dose
CGI=Clinical Global Impressions scale; CI=confidence interval; ER=extended release; HR=hazard ratio; PANSS=Positive and Negative Syndrome Scale.
*All patients diagnosed 1 to 5 years previously with ≥2 relapses requiring hospitalization.1
†The study was conducted in 26 countries, excluding the United States.1
‡Relapse defined by any of the following: psychiatric hospitalization; increase in the level of psychiatric care and an increase of 25% from baseline in PANSS total score (or an increase of 10 points if baseline score ≤40); deliberate self-injury; suicidal or homicidal ideation; violent behavior resulting in injury to another person or property damage; substantial clinical deterioration; required dose of antipsychotic exceeds the maximum approved dose.1
§At 24 months, 52 (14.8%) patients on INVEGA SUSTENNA® experienced a relapse vs 76 (20.9%) in the oral antipsychotic group.1
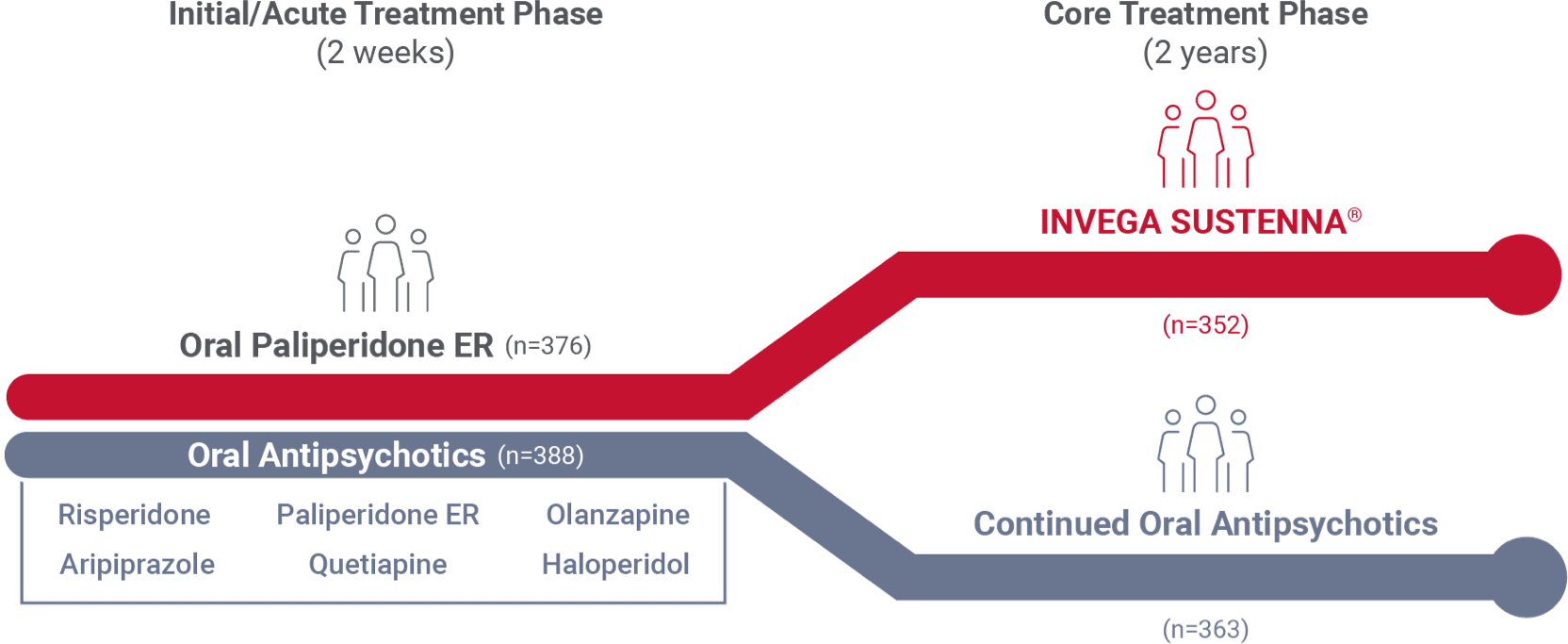
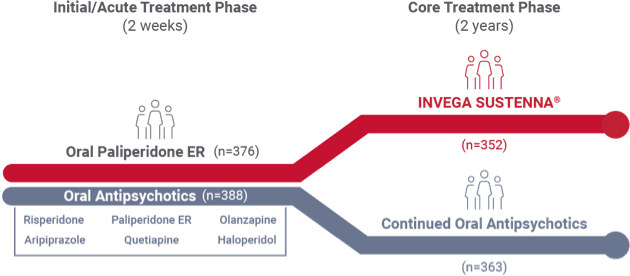
Recently diagnosed study design1
A 24-month, international study comparing INVEGA SUSTENNA® with 6 common oral antipsychotics in adult patients with recently diagnosed schizophrenia
- The study was not powered to compare the efficacy of INVEGA SUSTENNA® with that of individual oral antipsychotics
- The Prevention of Relapse With Oral Antipsychotics Versus Injectable Paliperidone Palmitate (PROSIPAL) study was a multicenter, randomized, prospective, active-controlled, open-label, rater-blinded, international, 24-month study in recently diagnosed (within 1–5 years) adult patients with schizophrenia (NCT01081769) that was conducted in 141 centers across 26 countries*
Key inclusion criteria
- Age: 18 to 65
- Acute episode of schizophrenia with PANSS total score of 70 to 120 at screening
- Diagnosis of schizophrenia (DSM-IV®) made 1 to 5 years previously
- History of ≥2 relapses requiring psychiatric hospitalization within preceding 24 months (may have included the current episode)
Key exclusion criteria
- Antipsychotic-naïve
- Considered by investigator to be treatment-resistant or unsuitable for treatment with an atypical oral antipsychotic or oral haloperidol monotherapy
- Received clozapine within past 3 months
- Use of LAIs within 3 injection cycles before screening
- Started a psychotherapy program within 2 months preceding baseline
- History of current symptoms of tardive dyskinesia
- History of neuroleptic syndrome
- Involuntary hospitalization
Study medications
2-week acute oral treatment phase
Patients randomized (1:1) to INVEGA SUSTENNA® or oral treatment arms entered a 2-week initial acute oral treatment phase
- INVEGA SUSTENNA® arm (n=376): Previous oral antipsychotic replaced with oral paliperidone ER (3-12 mg QD)
- Oral antipsychotic arm (n=388): Previous oral antipsychotic replaced with a different oral antipsychotic
- Percentages of patients receiving oral antipsychotics: aripiprazole (22.3%), paliperidone (21.2%), quetiapine (17.9%), risperidone (15.7%), olanzapine (13.5%), haloperidol (9.4%)
- Both groups: Previous oral antipsychotic was tapered over a maximum of 7 days
24-month core treatment phase
- INVEGA SUSTENNA® arm: Dosing was 234 mg intramuscular on Day 1 (deltoid), 156 mg on Day 8 (deltoid), 117 mg on Day 38 (deltoid or gluteal), then once monthly with flexible dosing of 39 mg to 234 mg (deltoid or gluteal)
- Substantial clinical deterioration: 6 (much worse) or 7 (very much worse) on the CGI change scale
- Oral antipsychotic arm: Continued on the same drug prescribed during the initial 2-week acute oral treatment phase; dosage adjustments were allowed throughout the study
CGI=Clinical Global Impressions scale; DSM-IV=Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; ER=extended-release; LAI=long-acting injectable; PANSS=Positive and Negative Syndrome Scale; QD=every day.
*The study was conducted in 26 countries, excluding the United States.
References: 1. Schreiner A, Aadamsoo K, Altamura AC, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169(1-3):393-399. 2. Data on file. Janssen Pharmaceuticals, Inc.
Back to Top