Click here to start your patients
on the path to just 2 DOSES A YEAR
- For US Healthcare Professionals Only
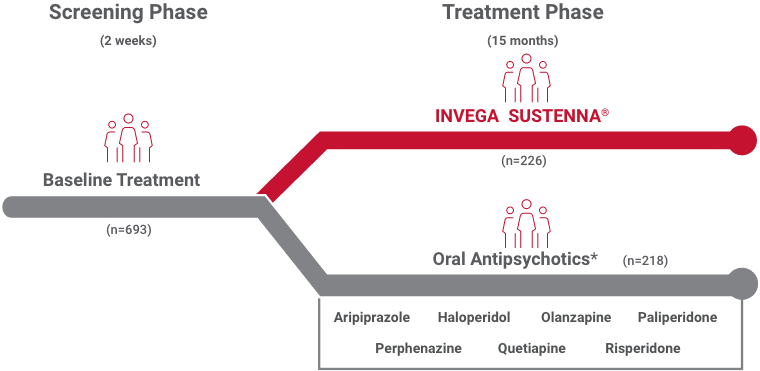
*Data from randomization until end of randomly assigned treatment (28 days after last injection of INVEGA SUSTENNA® or 1 day after last dose of oral antipsychotic). Population included adults diagnosed with schizophrenia within 5 years of study entry randomized to 1 of 7 oral antipsychotics.2
†The 7 oral antipsychotics included in the comparative arm accounted for 74% of oral schizophrenia treatment during the study period.3
‡Real-world as defined by patient selection and clinically meaningful outcome measures.
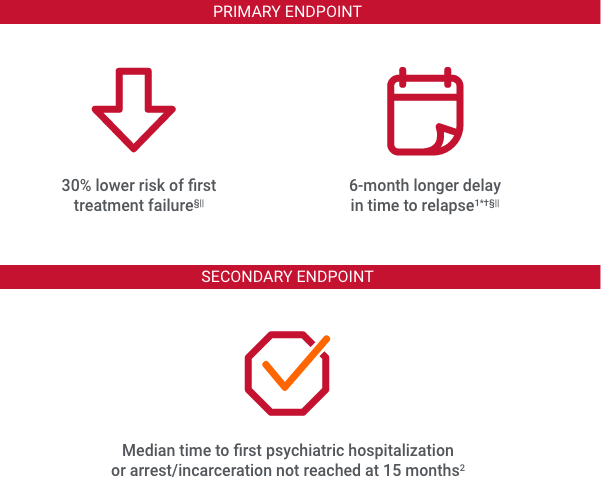
§Relapse was defined as time to first treatment failure.1
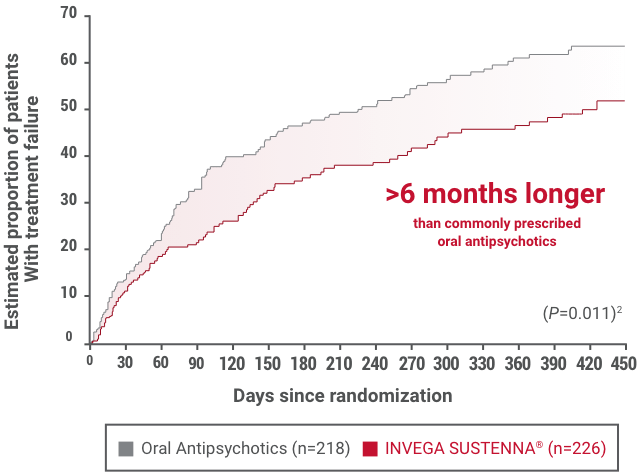
||Median time to first treatment failure in the INVEGA SUSTENNA® group was 416 days vs 226 days in the oral antipsychotic group.1
*Data from randomization until end of randomly assigned treatment (28 days after last injection of INVEGA SUSTENNA® or 1 day after last dose of oral antipsychotic). Population included adults diagnosed with schizophrenia within 5 years of study entry randomized to 1 of 7 oral antipsychotics.2
Select real-world† patient characteristics relevant to clinical practice‡ included adults living with schizophrenia often excluded from trials to compare how antipsychotics work in clinical practice2
59.5%—Patients with comorbid substance abuse
42.2 days—Mean time since release from last incarceration
†Real-world as defined by patient selection and clinically meaningful outcome measures.
‡The 7 oral antipsychotics included in the comparative arm accounted for 74% of oral schizophrenia treatment during the study period.3
Adult patients assigned to the INVEGA SUSTENNA® group were initiated with 2 injections in the deltoid muscle that were given approximately 1 week apart: 234 mg on Day 1 and 156 mg on Day 8 (±4 days)2
*Occasional dosing outside of the package insert range was allowed.
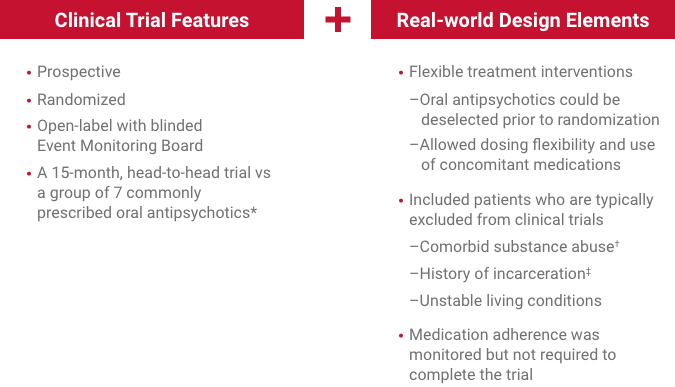
*The 7 oral antipsychotics included in the comparative arm accounted for 74% of oral schizophrenia treatment during the study period.3
†Except for patients who had abused intravenous drugs within 3 months of screening or had an opiate-dependence disorder (DSM-IV®).2
‡Patients must have been arrested ≥2 times in the previous 2 years, with ≥1 event leading to incarceration; released from most recent custody within 90 days of the screening visit.2
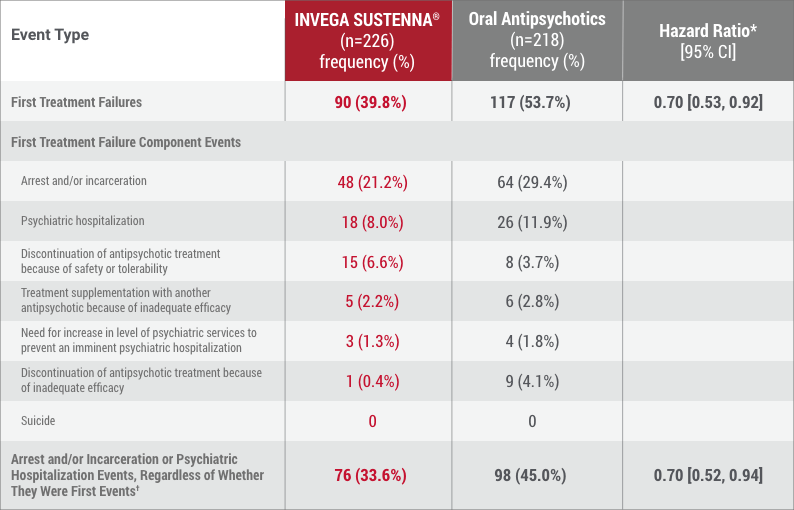
Time to first treatment failure, defined as 1 of the following:
Median time to first treatment failure in the INVEGA SUSTENNA® group was 416 days vs 226 days in the oral antipsychotic group.
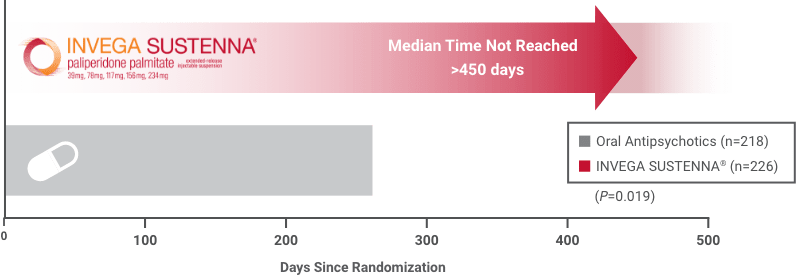
Median time to first psychiatric hospitalization or arrest/incarceration was not reached in the INVEGA SUSTENNA® group (>450 days). Median time to first psychiatric hospitalization or arrest/incarceration in the oral antipsychotic group was 274 days.2
QD=every day.
*Hazard ratio of INVEGA SUSTENNA® to oral antipsychotics based on Cox regression model for time-to-event analysis. Note that the hazard ratio did not appear constant throughout the trial.
†Analysis results, which incorporated relevant events collected after discontinuation for those who discontinued, were consistent with the results from the prespecified analysis of this secondary endpoint.
References: 1. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; July 2022. 2. Alphs L, Benson C, Cheshire-Kinney K, et al. Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board–blinded 15-month study. J Clin Psychiatry. 2015;76(5):554-561. 3. IMS Real World Data. May 2010-December 2013.