Click here to start your patients
on the path to just 2 DOSES A YEAR
- For US Healthcare Professionals Only
TARDIVE DYSKINESIA: The risk of developing tardive dyskinesia (TD) appears to increase with the duration of treatment and total cumulative dose but can develop after relatively brief treatment at low doses. Elderly female patients appeared to be at an increased risk for TD. If signs and symptoms of TD appear in a patient treated with INVEGA SUSTENNA®, drug discontinuation should be considered.1
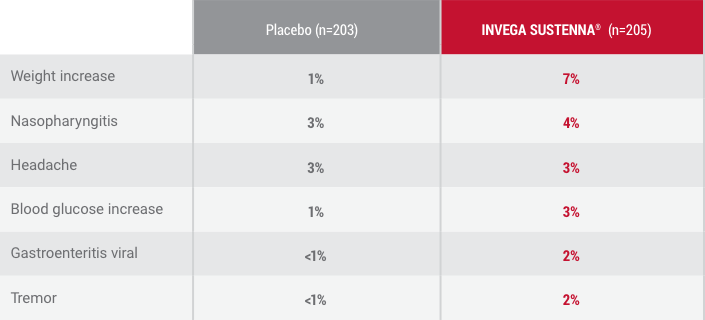
*Occurring in ≥2% of INVEGA SUSTENNA® patients and more frequently than placebo-treated patients during the double-blind phase.
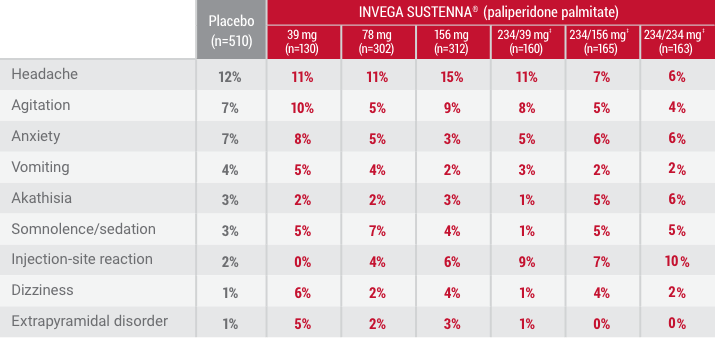
†Occurring in 5% of patients treated with INVEGA SUSTENNA®.
‡Initial deltoid injection of 234-mg followed by either 39-mg, 156-mg, or 234-mg every 4 weeks by deltoid or gluteal injection. Other dose groups (39-mg, 78-mg, and 156-mg) are from studies involving gluteal injection.1
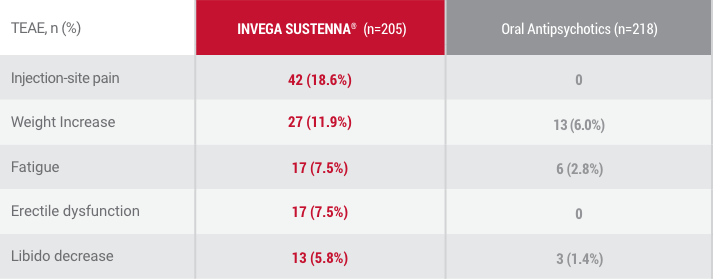
The safety of INVEGA SUSTENNA® was similar to that seen in previous schizophrenia double-blind, placebo-controlled trials1
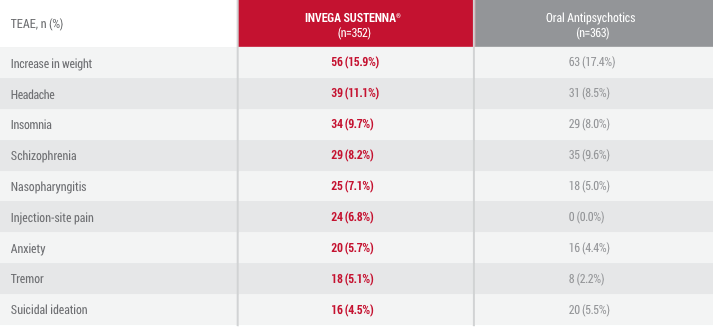
Data from randomization until end of randomly assigned treatment (28 days after last injection of INVEGA SUSTENNA® or 1 day after last dose of oral antipsychotic).

CYP3A4=cytocrome P450 3A4; P-gp=P-glycoprotein.
References: 1. INVEGA SUSTENNA® [Prescribing Information]. Titusville, NJ: Janssen Pharmaceuticals, Inc.; July 2022. 2. Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2-3):107-117. 3. Alphs L, Benson C, Cheshire-Kinney K, et al. Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76(5):554-561. 4. Schreiner A, Aadamsoo K, Altamura AC, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169:393-399.