Click here to start your patients
on the path to just 2 DOSES A YEAR
- For US Healthcare Professionals Only

This study was not powered to draw conclusions for individual reasons for relapse.
Indicated for the treatment of schizophrenia in adults.
The full constellation of symptoms and the relevant diagnostic criteria should be consulted and are available in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV®, or current version), where applicable.
*Population included adults diagnosed within 5 years of study entry.
†Defined as a 10-point increase (if the baseline score was ≤40) or ≥25% increase (if the baseline score was >40) in PANSS total score on 2 consecutive assessments.1,2
‡Defined as a score of ≥5 (if the maximum baseline score was ≤3) or ≥6 (if the maximum baseline score was 4) on 2 consecutive assessments of individual PANSS items: P1 (delusions), P2 (conceptual disorganization), P3 (hallucinatory behavior), P6 (suspiciousness/persecution), P7 (hostility), or G8 (uncooperativeness).1,2
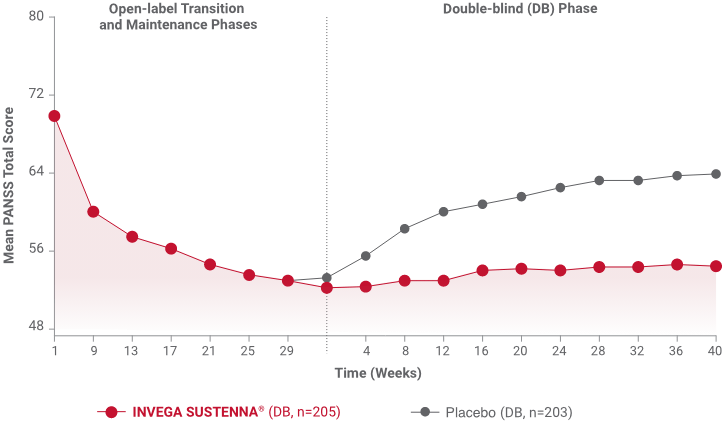
Mean PANSS total score* remained stable for patients on INVEGA SUSTENNA® while significantly worsening for patients on placebo during a longer-term study.2†
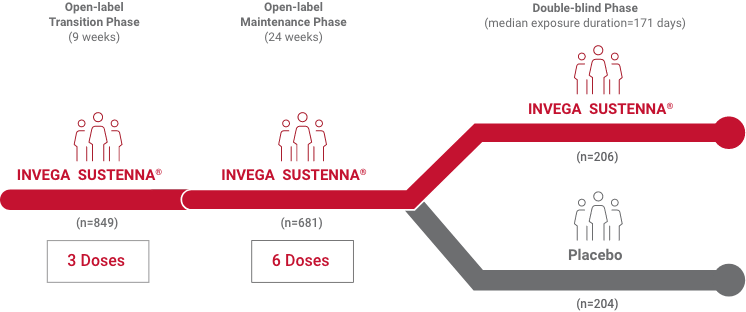
*Population included adults diagnosed within 5 years of study entry.
†Medium duration of total exposure in the INVEGA SUSTENNA® arm was 13 months.
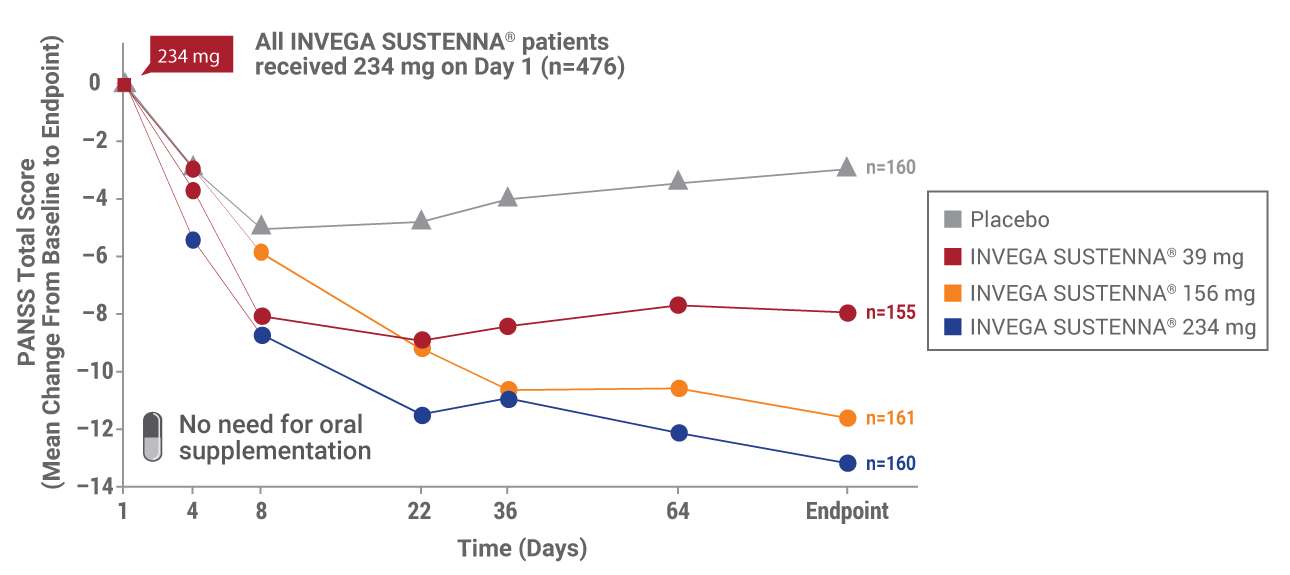
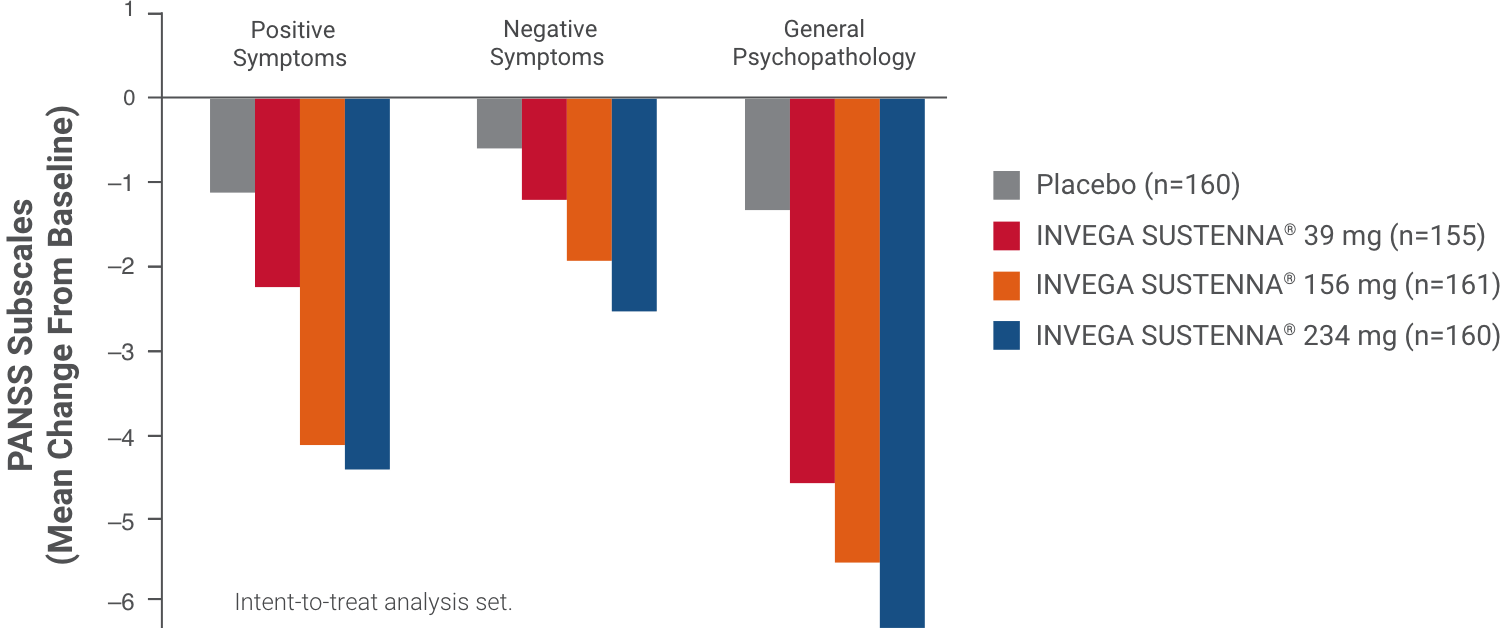
*Population included adults diagnosed within 5 years of study entry.
Results from a double-blind, randomized, placebo-controlled, fixed-dose, 13-week study of adult patients experiencing an acute exacerbation of schizophrenia. Patients were randomized to receive placebo or a 234-mg deltoid injection dose on Day 1, followed by a 39-mg, 156-mg, or 234-mg dose in either the deltoid or gluteal muscle on Day 8, and once monthly thereafter.1,4
The Positive and Negative Syndrome Scale (PANSS) is a 30-item scale that measures positive and negative symptoms of schizophrenia, as well as general psychopathology.1,5 The PANSS subscales can be used to assess symptom changes.5
PANSS=Positive and Negative Syndrome Scale